OCR A2 CHEMISTRY F324 and F325- 14th and 22nd June 2016- OFFICIAL THREAD
Scroll to see replies
Original post by AqsaMx
Not sure what colour the indicator would be, by saying its anion is blue, does this mean it's blue in acidic conditions??
Not sure what colour the indicator would be, by saying its anion is blue, does this mean it's blue in acidic conditions??
Anyone know what colours these pHs would give?
Original post by AqsaMx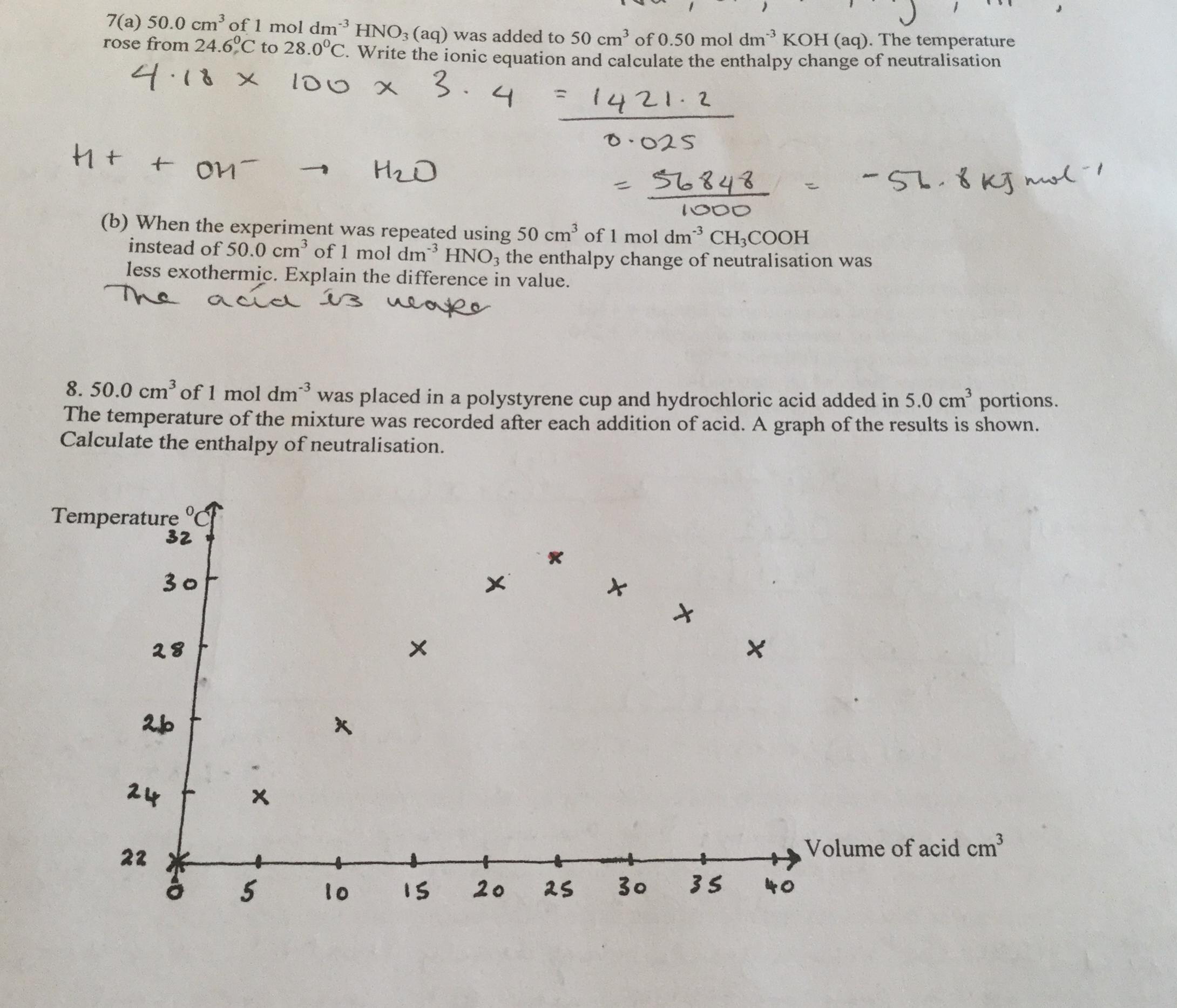
Does anyone know how to do (b) and question (8)
Seriously need help with these type of questions !
Does anyone know how to do (b) and question (8)
Seriously need help with these type of questions !
Anyone know how to do these questions??
Original post by AqsaMx
How do you know you have twice the number of moles of AgNO3? :/
When I work out the moles I get 2x10-3 Ag+ and 0.01 Cl-
When I work out the moles I get 2x10-3 Ag+ and 0.01 Cl-
0.01 is the number of moles in 250cm3, 25cm3 was taken from that and used in the titration so you have 10 times less moles, hence 0.001 moles of complex.
0.001:0.002
1:2
Original post by Serine Soul
I'm getting the same as thad333. Have you asked your tutors about it?
I mean the complexes it can be are
- [Cr(H2O)6]3+
- [Cr(H2O)5Cl]2+
- [Cr(H2O)4Cl2]+
And the compound and AgNO3 react in a 1:2 ratio, so 2xCl- , outside the complex ion, must be used up to react with the AgNO3
I feel like I'm missing something
I mean the complexes it can be are
- [Cr(H2O)6]3+
- [Cr(H2O)5Cl]2+
- [Cr(H2O)4Cl2]+
And the compound and AgNO3 react in a 1:2 ratio, so 2xCl- , outside the complex ion, must be used up to react with the AgNO3
I feel like I'm missing something
My teacher isn't the best at explaining things lol..
How do you know to multiply the moles of Cl by 2?
Original post by AqsaMx
How do you know you have twice the number of moles of AgNO3? :/
When I work out the moles I get 2x10-3 Ag+ and 0.01 Cl-
When I work out the moles I get 2x10-3 Ag+ and 0.01 Cl-
How many moles of the original compound is dissolved in 250cm3? How many moles will be in a 25cm3 portion of this solution?
It's good to challenge yourself, you never know when OCR will chuck in a crappy question (cough cough 'magic tang')
Original post by Serine Soul
It's good to challenge yourself, you never know when OCR will chuck in a crappy question (cough cough 'magic tang')
loool i hated that q, thats true i have a feeling that this yrs paper is going to be hard 😭
Posted from TSR Mobile
Original post by ranz
loool i hated that q, thats true i have a feeling that this yrs paper is going to be hard 😭
Posted from TSR Mobile
Posted from TSR Mobile
Probably will, being the last of the spec

Original post by AqsaMx
Really difficult question, anyone know how to do this?
Really difficult question, anyone know how to do this?
Hey, where are you getting these questions from?
 They look really good and I don't recognise them!
They look really good and I don't recognise them!What is everyone getting in past papers? I've left the 2014 papers for the week before the exams. But I've done all of the others + a few legacy papers (which I really recommend). I usually get 79-82 on F325 papers the first time I do them and anywhere from 50-54 on F324 papers. I really need harder questions to practise with the crap OCR can pull :I
Who is retaking unit 1 & 2 ocr chemsitry A ??
Original post by ToLiveInADream
What is everyone getting in past papers? I've left the 2014 papers for the week before the exams. But I've done all of the others + a few legacy papers (which I really recommend). I usually get 79-82 on F325 papers the first time I do them and anywhere from 50-54 on F324 papers. I really need harder questions to practise with the crap OCR can pull :I
F324: Usualy 90/90 UMS each time. The raw mark required for this varies quite considerably, from 50/60 in June 2015 to 59/60 in January 2013.
F325: I've only done two papers so far but 131/150 and 135/150 (UMS) so far.
Original post by AqsaMx
My teacher isn't the best at explaining things lol..
How do you know to multiply the moles of Cl by 2?
How do you know to multiply the moles of Cl by 2?
You don't have to double the moles of Chloride ions. You need to recognise that silver chloride has the formula AgCl.
Complex ion + AgNO3 -> AgCl
Notice that 1 Ag reacts with 1 Cl
but you have 2 times the moles of AgNO3 which you find out from the titration values. It tells you that they both react completely so the complex ion has to give up 2Cl- otherwise there would be a reactant left over.
Complex ion + 2AgNO3 -> 2AgCl
Which should make it obvious that the complex ion must give up 2 Cl-.
You're given the formula Cr(H2O)6Cl3 which can exist in a few ways which have already been shown. The reaction isn't ligand substitution so the 2Cl- lost must be attached outside the complex ion.
[Cr(H2O)6Cl]2+(Cl-)2 . (H2O)
Original post by thad33
You don't have to double the moles of Chloride ions. You need to recognise that silver chloride has the formula AgCl.
Complex ion + AgNO3 -> AgCl
Notice that 1 Ag reacts with 1 Cl
but you have 2 times the moles of AgNO3 which you find out from the titration values. It tells you that they both react completely so the complex ion has to give up 2Cl- otherwise there would be a reactant left over.
Complex ion + 2AgNO3 -> 2AgCl
Which should make it obvious that the complex ion must give up 2 Cl-.
You're given the formula Cr(H2O)6Cl3 which can exist in a few ways which have already been shown. The reaction isn't ligand substitution so the 2Cl- lost must be attached outside the complex ion.
[Cr(H2O)6Cl]2+(Cl-)2 . (H2O)
Complex ion + AgNO3 -> AgCl
Notice that 1 Ag reacts with 1 Cl
but you have 2 times the moles of AgNO3 which you find out from the titration values. It tells you that they both react completely so the complex ion has to give up 2Cl- otherwise there would be a reactant left over.
Complex ion + 2AgNO3 -> 2AgCl
Which should make it obvious that the complex ion must give up 2 Cl-.
You're given the formula Cr(H2O)6Cl3 which can exist in a few ways which have already been shown. The reaction isn't ligand substitution so the 2Cl- lost must be attached outside the complex ion.
[Cr(H2O)6Cl]2+(Cl-)2 . (H2O)
That makes a lot of sense thank you so much

Original post by ToLiveInADream
Hey, where are you getting these questions from?  They look really good and I don't recognise them!
They look really good and I don't recognise them!
 They look really good and I don't recognise them!
They look really good and I don't recognise them!They're from my college

Original post by marioman
F324: Usualy 90/90 UMS each time. The raw mark required for this varies quite considerably, from 50/60 in June 2015 to 59/60 in January 2013.
F325: I've only done two papers so far but 131/150 and 135/150 (UMS) so far.
F325: I've only done two papers so far but 131/150 and 135/150 (UMS) so far.
Push a bit harder for a couple more marks in F325. Just one or two raw marks can mean a lot of UMS in the upper boundaries for that paper. Then if you mess up a bit on F324 you can still comfortably get an A*.
Original post by ranz
how r ppl getting on with unit 4 tips?
Unit 4 as in F324?
F324 is a really nice paper as long as you know your stuff. Make sure you know the spec inside out and then practice papers! Practice lots of NMR and Synthesis. Make sure you know which groups can react with which substances so you can apply this in synthesis.
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Go ahead in time as far as possible
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- UNIQ 2024 Participants Thread
- OCR GCSE Geography A Paper 1 (J383/01) - 22nd May 2023 [Exam Chat]
- OCR A Level Business Paper 3 (H431/03) - 14th June 2023 [Exam Chat]
- OCR GCSE Geography B Paper 1 (J384/01) - 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 Foundation Tier (J248 01) - 22nd May 2023 [Exam Chat]
- OCR A-Level Biology A Paper 2 (H420/02) - 14th June 2024 [Exam Chat]
- Self-teaching Chemistry A-level (as a private candidate)?
- OCR B GCSE Chemistry Paper 3 Higher Tier (J258 03) - 22nd May 2023 [Exam Chat]
- Help polymers
- OCR GCSE Geography Geographical skills J383/03 - 14 Jun 2022 [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Help! How can I improve ?
Latest
Last reply 1 minute ago
March Riba part 2 Manchester vs SheffieldLast reply 3 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 7 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 13 minutes ago
Confused in undergrad final grade at University of GreenwichLast reply 29 minutes ago
Weidenfeld Hoffmann Trust (WHT) Scholarship Notification (2024-2025)Last reply 32 minutes ago
my student finance application isn't showing up when my parent tries to support itLast reply 50 minutes ago
Is it really such a bad thing to have an undefined relationshipLast reply 55 minutes ago
MPhil Advanced Computer Science Cambridge - 2024 EntryTrending
Last reply 6 days ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 6 days ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 6 days ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 6 days ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



