OCR Chemistry A Exam Thread (Breadth - May 27 2016 and Depth - June 10 2016)
Scroll to see replies
Original post by 11234
What did they mean by use a different symbol for oxygen and what did we have to do
It's a bit blurry but the top circle on the Oxygen I drew as a different symbol but I was not sure if it meant different as in a circle when uses crosses to represent it??
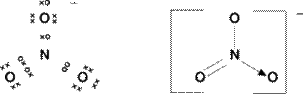
Original post by HarrisonGCSE
For the enthalpy change of reaction one i got confused - mainly regarding how many moles i had to divide the energy released by. For combustion u take the moles of the thing being combusted, for neutrilisation u take the moles of water, and for formation u take the compound. What on earth do u take for reaction?
Molar quantities in a given chemical equation
Original post by Jitesh
100cm3 I think you had to use (100g)
No. For that question they gave conc and vol. So you can work out the moles from that. And then using the moles, you can work out mass by doing moles x mr. That would given you the mass in g which is what you need to do q=mct
Original post by Fish40
Think I got same kJ but maybe different moles as I got -58.5 or something
That was so easy wtf
Posted from TSR Mobile
Posted from TSR Mobile
Original post by A Sajid
No. For that question they gave conc and vol. So you can work out the moles from that. And then using the moles, you can work out mass by doing moles x mr. That would given you the mass in g which is what you need to do q=mct
Are you sure? There's a very similar question to what came up on page 132 of the Oxford textbook (A-level edition)
Original post by 2kk2
how much marks will do u think you would lose if you missed out the - sign in the q value
1 normally
Original post by 456CJ
What did u guys get for water of crystilaztion
I got cr3cl10.18h20
I got cr3cl10.18h20
CrCl3.6H2O
Original post by HarrisonGCSE
For the enthalpy change of reaction one i got confused - mainly regarding how many moles i had to divide the energy released by. For combustion u take the moles of the thing being combusted, for neutrilisation u take the moles of water, and for formation u take the compound. What on earth do u take for reaction?
Molar quantities given in equation
Has anyone seen the unofficial mark scheme?
Original post by SGHD26716
CrCl3.6H2O
Was it not CrCl3.8H20 ? I think I remember getting 8 Oxygens
Might have been 6
Original post by Jitesh
It's a bit blurry but the top circle on the Oxygen I drew as a different symbol but I was not sure if it meant different as in a circle when uses crosses to represent it??


I did this but accidentally for some reason put Ni instead of N. How many marks would be lost?
Original post by SGHD26716
CrCl3.6H2O
Same

Original post by Fish40
I'm an idiot, didn't write a minus, so you divided your q by 0.075 mol and not 0.150mol, why?
Posted from TSR Mobile
Posted from TSR Mobile
You had to do 50cm^3 x 1.5moldm^3 not 100cm^3
Original post by Jitesh
Was it not CrCl3.8H20 ? I think I remember getting 8 Oxygens
Might have been 6
Might have been 6
I got 12 hydrogens and 6 oxygens and that goes 6 times for H2O. That what I did anyway.
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- GCSE Biology Study Group 2022-2023
- Grade Growth Chronicles | From C's to A's (23-24)
- OCR A GCSE Physics Paper 1 (Foundation Combined) J250/05- 25th May 2023 [Exam Chat]
- GCSE Exam Discussions 2023
- OCR A GCSE Physics Paper 2 (Foundation Combined) J250/06- 16th June 2023 [Exam Chat]
- OCR A GCSE Physics Paper 2 (Higher Combined) J250/12- 16th June 2023 [Exam Chat]
- OCR A GCSE Biology Paper 2 (Higher Combined) J250/08 - 9th June 2023 [Exam Chat]
- GCSE Exam Discussions 2024
- OCR A GCSE Biology Paper 2 (Foundation Combined) J250/02- 9th June 2023 [Exam Chat]
- OCR A GCSE Physics Paper 1 (Higher Combined) J250/11- 25th May 2023 [Exam Chat]
- A Level Exam Discussions 2023
- OCR A GCSE Biology Paper 1 (Foundation Combined) J250/01- 16th May 2023 [Exam Chat]
- OCR A GCSE Biology Paper 1 (Higher Combined) J250/07- 16th May 2023 [Exam Chat]
- GCSE Chemistry Study Group 2023/2024
- OCR GCSE Geography A Paper 1 (J383/01) - 22nd May 2023 [Exam Chat]
- I achieved 45 Distinctions in an Access course with the DistanceLearningCentre. AMA!
Latest
Posted 1 minute ago
Please check my creative writing first paragraph and give feed back on itLast reply 2 minutes ago
Medical doctor degree apprenticeship 2024Last reply 2 minutes ago
Is MathsFM French Alevels good for a CS degree at top uni (OX, UCL, Durham etc)Last reply 2 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 5 minutes ago
University of Portsmouth Dental Hygiene and Dental Therapy interviewLast reply 6 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 7 minutes ago
Degree level solicitor apprenticeshipsLast reply 8 minutes ago
Unit 6 website devolopment assignment 1 LEVEL 3 Btec ITLast reply 9 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 11 minutes ago
Official LSE Postgraduate Applicants 2024 ThreadLast reply 16 minutes ago
went from 3s to 9s with (literally) night before revision - ask me anythingGCSEs
30
Last reply 18 minutes ago
Official UCL Offer Holders Thread for 2024 entryTrending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



