OCR A2 CHEMISTRY F324 and F325- 14th and 22nd June 2016- OFFICIAL THREAD
Scroll to see replies
Original post by laplaplap
has anyone got any good nmr resources
What are you looking for?
Original post by thad33
Your first answer looks right to me.
Henderson-Hasselbalch equation:
pH = pKa + log(A-/HA)
3 = 4 + log(A-/HA)
log(A-/HA) = -1
So the ratio must be 1/10 as 10^-1 = 1/10
Your second answer would be the opposite so 10/1 as you need log(A-/HA) = 1.
I think anyway, I could be missing something.
Posted from TSR Mobile
Henderson-Hasselbalch equation:
pH = pKa + log(A-/HA)
3 = 4 + log(A-/HA)
log(A-/HA) = -1
So the ratio must be 1/10 as 10^-1 = 1/10
Your second answer would be the opposite so 10/1 as you need log(A-/HA) = 1.
I think anyway, I could be missing something.
Posted from TSR Mobile
Just tried this again, for the second one I still get 1/10 when i do the ratio of acid to conjugate base?
Original post by TeachChemistry
Here
Hi
Can you please explain how you have done this question. I really don't understand it. Thanks
Posted from TSR Mobile
Original post by ImNervous
Hi
Can you please explain how you have done this question. I really don't understand it. Thanks
Posted from TSR Mobile
Can you please explain how you have done this question. I really don't understand it. Thanks
Posted from TSR Mobile
1. Write half equation for generation of hydrogen in aqueous hydroxide
2. Write half equation for generation of oxygen in aqueous hydroxide
3. Multiply equations so that they both have the same number of electrons in them.
4. Combine half equations and cancel like terms.
Original post by AqsaMx
Could anyone explain 3??
I thought the weakest oxidising agent would be the strongest reducing agent and be the one with the most positive electrode potential?
Could anyone explain 3??
I thought the weakest oxidising agent would be the strongest reducing agent and be the one with the most positive electrode potential?
the weakest oxidising agent would be the strongest reducing agent and be the one with the most negative electrode potential
The negative sign tells you the half equation doesn't want to go from left to right as written
Original post by TeachChemistry
the weakest oxidising agent would be the strongest reducing agent and be the one with the most negative electrode potential
The negative sign tells you the half equation doesn't want to go from left to right as written
The negative sign tells you the half equation doesn't want to go from left to right as written
But if it's the strongest reducing agent then it goes from left to right ?
Oh never mind, I got it thanks !

(edited 7 years ago)
Original post by AqsaMx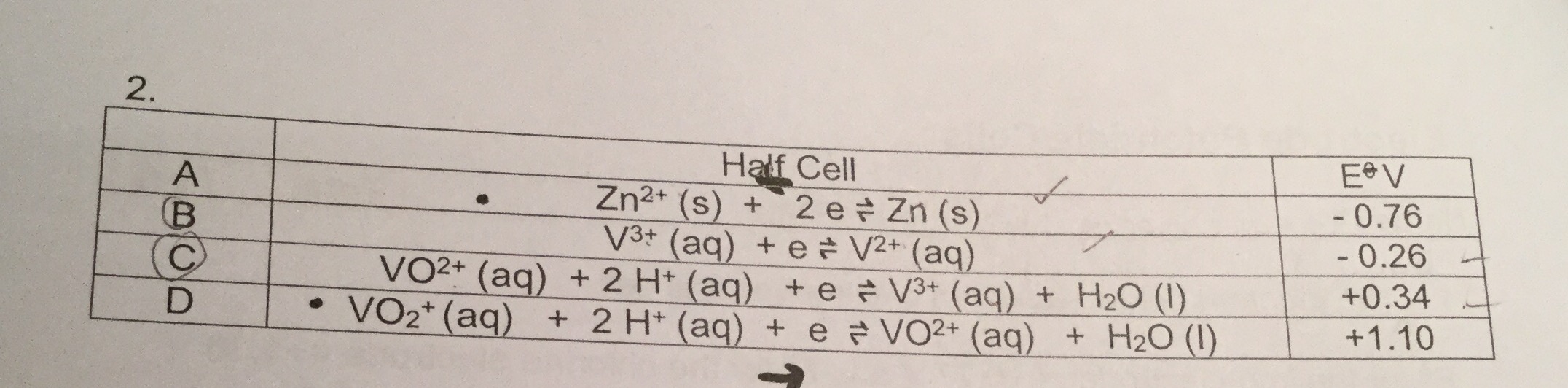
Anyone know how to work out the final species?
Attachment not found
Anyone know how to work out the final species?
V2+
Original post by TeachChemistry
V2+
Okay thank you !
Okay just did Jan f325 and got 80! However, the last question and the electrode potentials were confusing. Can someone help me understand how to answer question 7d. Here is a link:
http://www.ocr.org.uk/Images/144762-question-paper-unit-f325-01-equilibria-energetics-and-elements.pdf
http://www.ocr.org.uk/Images/144762-question-paper-unit-f325-01-equilibria-energetics-and-elements.pdf
Original post by ToLiveInADream
Okay just did Jan f325 and got 80! However, the last question and the electrode potentials were confusing. Can someone help me understand how to answer question 7d. Here is a link:
http://www.ocr.org.uk/Images/144762-question-paper-unit-f325-01-equilibria-energetics-and-elements.pdf
http://www.ocr.org.uk/Images/144762-question-paper-unit-f325-01-equilibria-energetics-and-elements.pdf
7d? Very tricky.
Anyone tried the Edexcel papers? Printed Jan 2016 Unit 4 and Unit 5. Which are more useful for our spec? 

Original post by Ss0
where can i find hard NMR questions? or if i can find any stretch and challenge chemistry questions in general?
http://webspectra.chem.ucla.edu//
http://www.chem.ucalgary.ca/courses/351/WebContent/spectroscopy/index.html
http://www-usr.rider.edu/~grushow/nmr/NMR_tutor/selftests/problems_fs_start.html
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch13/isp/index.html
There's more online if you look. I have used the first three and they're good. You just need to be careful not to go on the problems with additional spectra
Posted from TSR Mobile
Does anyone please have last years papers and mark schemes please???
Original post by AqsaMx
When drawing optical isomers, does it matter you order the groups around the chiral centre???
I don't really get your question sorry… If you're asking if the chiral carbon has to be in the middle then yes. IF you're asking about the way in which you draw the 3D lines connecting the 4 groups to central chiral carbon then the examiners generally accept two versions, the most common being the usual tetrahedral shape
//
(edited 7 years ago)
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Go ahead in time as far as possible
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- UNIQ 2024 Participants Thread
- OCR GCSE Geography A Paper 1 (J383/01) - 22nd May 2023 [Exam Chat]
- OCR GCSE Geography B Paper 1 (J384/01) - 22nd May 2023 [Exam Chat]
- OCR A Level Business Paper 3 (H431/03) - 14th June 2023 [Exam Chat]
- OCR A-Level Biology A Paper 2 (H420/02) - 14th June 2024 [Exam Chat]
- OCR B GCSE Chemistry Paper 3 Higher Tier (J258 03) - 22nd May 2023 [Exam Chat]
- Help polymers
- Self-teaching Chemistry A-level (as a private candidate)?
- OCR GCSE Geography Geographical skills J383/03 - 14 Jun 2022 [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Help! How can I improve ?
- How long did you get the offer from Warwick?
Latest
Trending
Last reply 2 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 2 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



