OCR A2 CHEMISTRY F324 and F325- 14th and 22nd June 2016- OFFICIAL THREAD
Scroll to see replies
I can't tell if I'm confusing myself here but how do we know which peak the protons are from in proton NMR? (The ones I circled) I don't even know how to ask this question properly but hoping someone understands me lol 
Posted from TSR Mobile
Posted from TSR Mobile
Original post by Saywhatyoumean
I can't tell if I'm confusing myself here but how do we know which peak the protons are from in proton NMR? (The ones I circled) I don't even know how to ask this question properly but hoping someone understands me lol 
Posted from TSR Mobile
Posted from TSR Mobile
You only circled one proton...
Original post by alow
You only circled one proton...
Yeah but I mean which peak would that proton be for the chemical shift values? The H-C-O or the H-C=O?
Posted from TSR Mobile
Original post by Saywhatyoumean
Yeah but I mean which peak would that proton be for the chemical shift values? The H-C-O or the H-C=O?
Posted from TSR Mobile
Posted from TSR Mobile
What do you mean?
Original post by alow
What do you mean?
I wish I knew lol hang on let me find the question
So when I'm drawing the spectrum, why is the CH2 singlet peak between 3.3-4.3ppm instead of ~2-3ppm?
Posted from TSR Mobile
Original post by Saywhatyoumean
I wish I knew lol hang on let me find the question 

So when I'm drawing the spectrum, why is the CH2 singlet peak between 3.3-4.3ppm instead of ~2-3ppm?
Posted from TSR Mobile
So when I'm drawing the spectrum, why is the CH2 singlet peak between 3.3-4.3ppm instead of ~2-3ppm?
Posted from TSR Mobile
The CH2 is deshielded by virtue of its proximity to two electron withdrawing groups.
Original post by alow
The CH2 is deshielded by virtue of its proximity to two electron withdrawing groups.
surely it's because the carbon atom attached to the 2Hs is also attached to an oxygen.Therefore the corresponding chemical shift would be 3.3-4.3,which represents the HC-O proton signal.
Original post by suibster
surely it's because the carbon atom attached to the 2Hs is also attached to an oxygen.Therefore the corresponding chemical shift would be 3.3-4.3,which represents the HC-O proton signal.
The chemical shift of an environment isn't only determined by one of the groups it is attached to...
Original post by suibster
No it's determined by the functional group the c is attached to.
I don't think you understood what I said.
Original post by AqsaMx
I got 7.8g, think I did it wrong though..
I got 7.79 but I used a really old calculater so I couldn't store the values.
Posted from TSR Mobile
Hi, can somebody clarify how you make a diazonium ion (with a balanced equation) please?
In the textbook, pg. 39, it says you need 2HCl but I think this is incorrect?
In the textbook, pg. 39, it says you need 2HCl but I think this is incorrect?
Original post by chuckster111
Hi, can somebody clarify how you make a diazonium ion (with a balanced equation) please?
In the textbook, pg. 39, it says you need 2HCl but I think this is incorrect?
In the textbook, pg. 39, it says you need 2HCl but I think this is incorrect?
Hey you need 1 HCl to make HNO2 and another to make the ion
Original post by chuckster111
Hi, can somebody clarify how you make a diazonium ion (with a balanced equation) please?
In the textbook, pg. 39, it says you need 2HCl but I think this is incorrect?
In the textbook, pg. 39, it says you need 2HCl but I think this is incorrect?
Yes, that equation is wrong, it's only 1 HCl. If you wanted to write a full equation, its:
phenylamine + NaNO2 + 2HCl ----> diazonium ion + NaCl + 2H2O
Original post by rory58824
Yes, that equation is wrong, it's only 1 HCl. If you wanted to write a full equation, its:
phenylamine + NaNO2 + 2HCl ----> diazonium ion + NaCl + 2H2O
phenylamine + NaNO2 + 2HCl ----> diazonium ion + NaCl + 2H2O
Ok, so if you want the overall equation, it's the above? (Wouldn't it be the diazonium salt rather than ion btw?).
And if you do it in two steps, it would be:
1. NaNO2 + HCl ----> NaCl + HNO2
2. phenylamine + HNO2 + HCl ---> diazonium salt + 2H2O
?
Original post by chuckster111
Ok, so if you want the overall equation, it's the above? (Wouldn't it be the diazonium salt rather than ion btw?).
And if you do it in two steps, it would be:
1. NaNO2 + HCl ----> NaCl + HNO2
2. phenylamine + HNO2 + HCl ---> diazonium salt + 2H2O
?
And if you do it in two steps, it would be:
1. NaNO2 + HCl ----> NaCl + HNO2
2. phenylamine + HNO2 + HCl ---> diazonium salt + 2H2O
?
Isn't my attachment loading?

Original post by chuckster111
Ok, so if you want the overall equation, it's the above? (Wouldn't it be the diazonium salt rather than ion btw?).
And if you do it in two steps, it would be:
1. NaNO2 + HCl ----> NaCl + HNO2
2. phenylamine + HNO2 + HCl ---> diazonium salt + 2H2O
?
And if you do it in two steps, it would be:
1. NaNO2 + HCl ----> NaCl + HNO2
2. phenylamine + HNO2 + HCl ---> diazonium salt + 2H2O
?
I've noticed the textbook changes between ion and salt (maybe someone who knows more could clarify this lol). Probably best to use salt, my bad.
And yep, that looks fine.
(edited 7 years ago)
Original post by Serine Soul
Isn't my attachment loading? 

Hi, sorry I'm on my phone I didn't realise there was an attachment!

Thanks for that

Original post by rory58824
I've noticed the textbook changes between ion and salt (maybe someone who knows more could clarify this lol)
And yep, that looks fine.
And yep, that looks fine.
It is a salt if another negative ion is with it, like Chlorine. It then becomes Diazonium chloride which is a salt
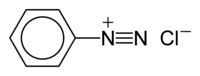
If it is just the first part by itself, like below, then it is a diazonium ion
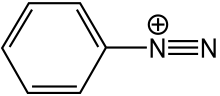
Original post by rory58824
I've noticed the textbook changes between ion and salt (maybe someone who knows more could clarify this lol)
And yep, that looks fine.
And yep, that looks fine.
I haven't looked at the textbook for this topic (too simplified) but remember that the diazonium salt is essentially the diazonium ion with a Cl-
In synthetic routes etc on a question paper, it often says to ignore the presence of the Cl- ion next to the diazonium ion.
When writing the full reaction in itself though, I'd go for the HNO2 + HCl route and draw the salt as the product rather than just the ion
(edited 7 years ago)
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Go ahead in time as far as possible
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- UNIQ 2024 Participants Thread
- OCR GCSE Geography A Paper 1 (J383/01) - 22nd May 2023 [Exam Chat]
- OCR GCSE Geography B Paper 1 (J384/01) - 22nd May 2023 [Exam Chat]
- OCR A Level Business Paper 3 (H431/03) - 14th June 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 Foundation Tier (J248 01) - 22nd May 2023 [Exam Chat]
- OCR A-Level Biology A Paper 2 (H420/02) - 14th June 2024 [Exam Chat]
- Self-teaching Chemistry A-level (as a private candidate)?
- OCR B GCSE Chemistry Paper 3 Higher Tier (J258 03) - 22nd May 2023 [Exam Chat]
- Help polymers
- OCR GCSE Geography Geographical skills J383/03 - 14 Jun 2022 [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Help! How can I improve ?
Latest
Last reply 1 minute ago
Official UCL Offer Holders Thread for 2024 entryLast reply 1 minute ago
LSE Economic history departmentLast reply 2 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2916
Last reply 2 minutes ago
National Audit Office Apprenticeship 2024Last reply 4 minutes ago
Amazon Project management apprenticeship 2024Last reply 8 minutes ago
Why is the political left now censorious and authoritarian??Last reply 19 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 20 minutes ago
University of Oxford 2025 Undergraduate Applicants Official ThreadLast reply 24 minutes ago
Official: University of Leicester A199 (foundation year) 2024 entry applicantsPosted 24 minutes ago
University of Surrey academics back no confidence vote as 'morale is very low'Trending
Last reply 6 days ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 6 days ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 6 days ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 6 days ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



