OCR Chemistry A - AS in Depth - 2015 (new spec) UNOFFICIAL MARK SCHEME
Scroll to see replies
Idk how that C-O-H question was worth 4 marks
104.5 (1)
2 bond pairs, 2 lone pairs (1)
Lone pair repels more than bond pair (1)
Then I put it should be 109.5 if it was bond pair but each lone pair reduces the angle by 2.5, so the 2 lone pairs 109.5-5=104.5
104.5 (1)
2 bond pairs, 2 lone pairs (1)
Lone pair repels more than bond pair (1)
Then I put it should be 109.5 if it was bond pair but each lone pair reduces the angle by 2.5, so the 2 lone pairs 109.5-5=104.5
What do we think grade boundaries will be? And are they using UMS?
Original post by alkaline.
okay ill put what i can remember in terms of workings spoilers - I can't remember everything lol I'll try
also I don't remember what numbers each question was but I've tried to group them into colour schemes for whole questions
if you remember anything additional please do say and I'll edit it in.
also I don't remember what numbers each question was but I've tried to group them into colour schemes for whole questions
if you remember anything additional please do say and I'll edit it in.
I don't think you need to go to the trouble of writing all these calculations out! That user obviously hasn't done the AS exam and his/ her school is probably using the exam we did today as a mock for their predicted grades. So upload if you don't care about that, I just wanted to warn you as OCR do want their papers secure and not leaked.
Answers are still gonna spill out, it's just that you shouldn't feel obliged to write out all the calculations, you get me?
Hope the exam went well for you! I'm noticing all these silly errors I made :/
Original post by neek101
What did everyone get for the structure of C for the last question?
I got it wrong but the right answer was 2-methylpropanoic acid
Original post by simply_a_ Δ
I don't think you need to go to the trouble of writing all these calculations out! That user obviously hasn't done the AS exam and his/ her school is probably using the exam we did today as a mock for their predicted grades. So upload if you don't care about that, I just wanted to warn you as OCR do want their papers secure and not leaked.
Answers are still gonna spill out, it's just that you shouldn't feel obliged to write out all the calculations, you get me?
Hope the exam went well for you! I'm noticing all these silly errors I made :/
Answers are still gonna spill out, it's just that you shouldn't feel obliged to write out all the calculations, you get me?
Hope the exam went well for you! I'm noticing all these silly errors I made :/
oh crap what?
ppl make these threads all the time though if im violating the law ppl tell me cause I don't wanna get nullified as levels...
yeah I cba to put the calculations lol
thank you I think it went okay, I also made stupid errors dw
Original post by alkaline.
oh crap what?
ppl make these threads all the time though if im violating the law ppl tell me cause I don't wanna get nullified as levels...
yeah I cba to put the calculations lol
thank you I think it went okay, I also made stupid errors dw
ppl make these threads all the time though if im violating the law ppl tell me cause I don't wanna get nullified as levels...
yeah I cba to put the calculations lol
thank you I think it went okay, I also made stupid errors dw
no don't worry, you won't get disqualified! It's just that ocr may ask you to take this down. Don't worry, the a level chemsitry teacher who posted his answers to the breadth paper also just got told to delete it, he didn't get sacked haha!
OCR know that their papers will get leaked so they can't really do much to control it, so it's just up to us to respect their wishes
Original post by 4nonymous
For the draw the diagram for the equipment and experiment, instead of a gas syringe I did a measuring cylinder in a tray of water cos thats how we did it in class?
That's what was written in the Biology textbook so is valid but the Chemistry textbook says you should use a gas syringe so

Original post by simply_a_ Δ
no don't worry, you won't get disqualified! It's just that ocr may ask you to take this down. Don't worry, the a level chemsitry teacher who posted his answers to the breadth paper also just got told to delete it, he didn't get sacked haha!
OCR know that their papers will get leaked so they can't really do much to control it, so it's just up to us to respect their wishes
OCR know that their papers will get leaked so they can't really do much to control it, so it's just up to us to respect their wishes
I understand, I mean I just wanted to discuss the answers I'm not trying to leak the papers lol
plus they were really annoying this year they didn't even post the answers to the practise questions until some weeks before the exams?!
anyway i don't get what the big deal is they'll release the papers next year anyway? what's leaking really
There is no such thing as 2-methylpropanoic acid. The compound is methylpropanoic acid.
If the stem is 'prop', any alkyl groups must be attached to the second carbon atom- otherwise the stem changes to 'but'.
The '2' is therefore redundant.
(Not sure if marks will be docked for this.)
If the stem is 'prop', any alkyl groups must be attached to the second carbon atom- otherwise the stem changes to 'but'.
The '2' is therefore redundant.
(Not sure if marks will be docked for this.)
The question about the graph, was describe and explain the rate of reaction. If the mass was decreasing surly the rate of reaction was increasing?
Yeah that's what I thought too!
Original post by Tom malikk
The question about the graph, was describe and explain the rate of reaction. If the mass was decreasing surly the rate of reaction was increasing?
why is this apparatus not suitable for oxidising a secondary alcohol?
Its because some of the reactants are volatile so may evaporate before reacting, which is why you need to use reflux apparatus.
Its because some of the reactants are volatile so may evaporate before reacting, which is why you need to use reflux apparatus.
Original post by alkaline.
yo we don't have one yet.
this is what I remember pls feel free to correct and also add missed questions.
the colour scheme is to try and separate whole questions.
draw the apparatus you'd use:
I put a clamp stand with a syringe on the left, and a clamp stand holding a test tube with metal and water in with the bung connected to the syringe.
OR
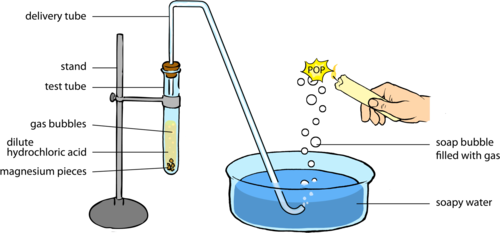
the unknown metal: calcium
percentage yield: 67.4%
explain oxidation of magnesium in terms of electrons (1 mark)
oxidation of magnesium is loss of two electrons.
electron configuration of a magnesium atom
1s2 2s2 2p6 3s2
Magnesium vs Silicon
Magnesium = giant metallic, metallically bonded = electrostatic attraction between negatively charged delocalised sea of electrons and positively charged Mg ions.
Silicon = giant molecular, covalently bonded (electrostatic attraction between shared pairs of electron and the nuclei of bonding atoms), forming a lattice.
the difference in melting point - p4 and cl2 - on the graph chlorine was lower
I put: they are both covalently bonded, butchlorine is a smaller molecule, and so has weaker london forces therefore less energy is required to break the intermolecular forces therefore lower melting point.
second ionisation energy for strontium inc state symbols
Sr+ (g) --> Sr2+ (g) + e-
a question to do with explaining ionisation energy trend down group 2 but I can't remember btwn which elements
nuclear charge increases down the group however this is outweighed by increase in shielding and also increase in atomic radius, therefore there is less nuclear attraction (between nucleus and outer electrons) so outer electrons are more easily lost/less energy is required to remove them therefore lower first ionisation energy.
mass of strontium carbonate = 1.845g
rates question: why was there loss in mass or something?
I put cause the reactants were getting used up and (2 mol of gas given off)
describe and explain the trend in the graph up to 200s.
mass rapidly decreases as time goes on = high ROR
explain why enthalpy change of formation for NO was exothermic in terms of bonds
I put because more bonds made than were broken and making bonds releases energy whereas bond breaking uses up energy.
enthalpy profile diagram: endothermic
bottom left = reactants = N2 + 0.5 O2
top right = products = n20
pv=nrt question: (in standard form) 4.46*10^6 Pa
show NO catalyses the break down of ozone:NO• + O3 -> NO2• + O2NO2• + O -> NO• + O2
mechanism for the reaction of compound A an alkene with a hydrogen halide (HBr)
show the mechanism, the two different products, explain which of two would form more of.
unsymmetrical alkene CH3HC=CCH3CH3 , basically the second possible product just had the H and Br on the resulting haloalkane switched around, more of the tertiary halogenoalkane would be formed because it's more stable.
why can't compound A (alkene) exhibit E/Z isomerism?
because it doesn't have 2 different groups on each carbon of the double bond.
Z isomer for the alkene:
Z pent-2-ene
structural isomers: 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
why is it a secondary carbocation?
because the halogen is attached to the carbon that is attached to 2 alkyl groups
write equation for butan-2-ol oxidising
I drew out: butan-2-ol + [O] - butanone + h20
bond angle around the oxygen in the butan-2-ol (3 marks)
104.5
reason: 2 bonding pairs and 2 lone pairs around the central atom, electron pairs repel to maximum separation = non-linear shape.why is this apparatus not suitable for oxidising a secondary alcohol?
i didn’t know this but I put: because nowhere to separately collect the products, reflux apparatus would be better.
aluminium sulfide and water forming aluminium nitrate and hydrogen sulfide
Al2S3 + 6H2O -> 2Al(OH)3 + 3 H2S
last question (mass spectrum/ infrared red) conspiracy as it currently stands = methylpropanoic acid
this is what I remember pls feel free to correct and also add missed questions.
the colour scheme is to try and separate whole questions.
draw the apparatus you'd use:
I put a clamp stand with a syringe on the left, and a clamp stand holding a test tube with metal and water in with the bung connected to the syringe.
OR

the unknown metal: calcium
percentage yield: 67.4%
explain oxidation of magnesium in terms of electrons (1 mark)
oxidation of magnesium is loss of two electrons.
electron configuration of a magnesium atom
1s2 2s2 2p6 3s2
Magnesium vs Silicon
Magnesium = giant metallic, metallically bonded = electrostatic attraction between negatively charged delocalised sea of electrons and positively charged Mg ions.
Silicon = giant molecular, covalently bonded (electrostatic attraction between shared pairs of electron and the nuclei of bonding atoms), forming a lattice.
the difference in melting point - p4 and cl2 - on the graph chlorine was lower
I put: they are both covalently bonded, butchlorine is a smaller molecule, and so has weaker london forces therefore less energy is required to break the intermolecular forces therefore lower melting point.
second ionisation energy for strontium inc state symbols
Sr+ (g) --> Sr2+ (g) + e-
a question to do with explaining ionisation energy trend down group 2 but I can't remember btwn which elements
nuclear charge increases down the group however this is outweighed by increase in shielding and also increase in atomic radius, therefore there is less nuclear attraction (between nucleus and outer electrons) so outer electrons are more easily lost/less energy is required to remove them therefore lower first ionisation energy.
mass of strontium carbonate = 1.845g
rates question: why was there loss in mass or something?
I put cause the reactants were getting used up and (2 mol of gas given off)
describe and explain the trend in the graph up to 200s.
mass rapidly decreases as time goes on = high ROR
explain why enthalpy change of formation for NO was exothermic in terms of bonds
I put because more bonds made than were broken and making bonds releases energy whereas bond breaking uses up energy.
enthalpy profile diagram: endothermic
bottom left = reactants = N2 + 0.5 O2
top right = products = n20
pv=nrt question: (in standard form) 4.46*10^6 Pa
show NO catalyses the break down of ozone:NO• + O3 -> NO2• + O2NO2• + O -> NO• + O2
mechanism for the reaction of compound A an alkene with a hydrogen halide (HBr)
show the mechanism, the two different products, explain which of two would form more of.
unsymmetrical alkene CH3HC=CCH3CH3 , basically the second possible product just had the H and Br on the resulting haloalkane switched around, more of the tertiary halogenoalkane would be formed because it's more stable.
why can't compound A (alkene) exhibit E/Z isomerism?
because it doesn't have 2 different groups on each carbon of the double bond.
Z isomer for the alkene:
Z pent-2-ene
structural isomers: 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
why is it a secondary carbocation?
because the halogen is attached to the carbon that is attached to 2 alkyl groups
write equation for butan-2-ol oxidising
I drew out: butan-2-ol + [O] - butanone + h20
bond angle around the oxygen in the butan-2-ol (3 marks)
104.5
reason: 2 bonding pairs and 2 lone pairs around the central atom, electron pairs repel to maximum separation = non-linear shape.
Spoiler
i didn’t know this but I put: because nowhere to separately collect the products, reflux apparatus would be better.
aluminium sulfide and water forming aluminium nitrate and hydrogen sulfide
Al2S3 + 6H2O -> 2Al(OH)3 + 3 H2S
last question (mass spectrum/ infrared red) conspiracy as it currently stands = methylpropanoic acid
Another question was that if the group 2 metal was replaced with another one further down group in the reaction with the acid, will the volume increase, decrease or stay the same? I put it will decrease because the molar mass would increase and so the moles would decrease causing the volume to decrease.
There was another part of the question above this asking what group 2 metal is reacting, I got calcium.
(edited 7 years ago)
Original post by Alishajani10
Yeah that's what I thought too!
Original post by Tom malikk
The question about the graph, was describe and explain the rate of reaction. If the mass was decreasing surly the rate of reaction was increasing?
In the first few seconds the rate of reaction is fastest due to presence of the a lot of reactants. However rate of reaction decrease as the reactants get used up and less reactants are left to react (less Likely collision)
Value is negative because mass is decreasing so the graph goes downwards. This means the gradient will be negative.
Sorry I'm not the best at explaining but I've tried

(edited 7 years ago)
Original post by alkaline.
I understand, I mean I just wanted to discuss the answers I'm not trying to leak the papers lol
plus they were really annoying this year they didn't even post the answers to the practise questions until some weeks before the exams?!
anyway i don't get what the big deal is they'll release the papers next year anyway? what's leaking really
plus they were really annoying this year they didn't even post the answers to the practise questions until some weeks before the exams?!
anyway i don't get what the big deal is they'll release the papers next year anyway? what's leaking really
Yes at the end of the day it doesn't matter

I used the syringe method too! I hope its ok to do so.
Original post by alkaline.
thanks everyone for contributions so far!
also that first diagram I swear they said they wanted to measure the volume that's why I put a syringe, originally I had put the image you've posted :/
I said really similar like
weigh the beaker, add the acid weigh again, weigh oxide and add it, start stopwatch as soon as you add the oxide
i didn't say specifically measure the mass of src03 because the graph was (regants + container)
except i said record reading on the scale every 10 seconds
I think I was wrong too though.
also that first diagram I swear they said they wanted to measure the volume that's why I put a syringe, originally I had put the image you've posted :/
I said really similar like
weigh the beaker, add the acid weigh again, weigh oxide and add it, start stopwatch as soon as you add the oxide
i didn't say specifically measure the mass of src03 because the graph was (regants + container)
except i said record reading on the scale every 10 seconds
I think I was wrong too though.
Quick Reply
Related discussions
- OCR A biology (a level)
- AS Physics 2023 OCR A
- 1000+ A2-Level Biology Exam Questions
- How to get A* at OCR A level chemistry?
- OCR A-level Computer Science Paper 2 (H446/02) - 19th June 2023 [Exam Chat]
- AS/A Level Chemistry Study Group 2023/2024
- Revising for A level mocks
- Chemistry A-level
- URGENT!!!!!!!!!most effective way to study biology chemistry A level
- Failure
- Study Buddy
- Business Edexcel
- what website gives the content that's most accurate for OCR biology?
- OCR B A-level Physics Paper 1 Advancing Physics (H557/01) - 24th May 2023 [Exam Chat]
- Writing prompts in HK public exam
- Edexcel A-Level Chem Paper 1 Advanced Inorganic and Physical Chemistry [Exam Chat]
- How do I study for A Level physics ‘Explain’ questions?
- oxfordAQA chemistry papers
- OCR A LEVEL PHYSICS A paper 2 unofficial mark scheme
- GCSE Diary
Latest
Last reply 6 minutes ago
The Official Cambridge Applicants for 2024 Entry ThreadLast reply 6 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 7 minutes ago
Amazon Apprenticeships 2024Last reply 10 minutes ago
Executive Officer IMIU Remote Operations HubLast reply 11 minutes ago
fujitsu degree apprenticeship 2024Last reply 13 minutes ago
Can I self study the entirety of CAIE A Level Further Maths in one year?Last reply 16 minutes ago
Loughborough or Cardiff for architecture?Last reply 17 minutes ago
Official University of Bristol Applicant Thread for 2024Last reply 17 minutes ago
The Official Aberystwyth Applicants for 2024 Entry ThreadTrending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



