Unit-4 - Which (Acid-Base) indicator should I use?
Titrating ethanoic acid with 0.1mol dm-3 sodium hydroxide
Can someone tell me step-by-step on how to solve this question?
NOTE: I have the data booklet (A-level U4)
Can someone tell me step-by-step on how to solve this question?
NOTE: I have the data booklet (A-level U4)
Original post by Zahid~
Is there no other information in the question?
No, it's a MCQ question.
A) Thymol blue (acid)
B) Bromothymol blue
C) Thymol blue (base)
D) Alizarin yellow R
For strong acids it's commonly Phenolpthalein. Mainly cause estimated pH range for strong acid weak base graph a vertical region between around 7-10.5 is given, and the pH range of the indicator should correspond to the vertical region.
But if further information is given, we can give a more accurate indicator.
But if further information is given, we can give a more accurate indicator.
Original post by ihaspotato
No, it's a MCQ question.
A) Thymol blue (acid)
B) Bromothymol blue
C) Thymol blue (base)
D) Alizarin yellow R
A) Thymol blue (acid)
B) Bromothymol blue
C) Thymol blue (base)
D) Alizarin yellow R
Is Thymol blue (base) the answer?
Original post by AvWOW
Is Thymol blue (base) the answer?
CORRECT! can you please tell the steps you did to get to that conclusion?
(edited 7 years ago)
Original post by ihaspotato
CORRECT! but but hooow?!??
I gave the explanation ^. I wrote in terms of phenolpthalein, but it applies to Thymol Blue (base)
Basically vertical region for weak acid strong base is around 7-10.5 and the pH range for Thymol Blue (base) is 8.0-9.6 (from the data booklet) which is WITHIN the range
 so giving a clear colour change
so giving a clear colour changeOriginal post by AvWOW
I gave the explanation ^. I wrote in terms of phenolpthalein, but it applies to Thymol Blue (base)
Basically vertical region for weak acid strong base is around 7-10.5 and the pH range for Thymol Blue (base) is 8.0-9.6 (from the data booklet) which is WITHIN the range so giving a clear colour change
so giving a clear colour change
Basically vertical region for weak acid strong base is around 7-10.5 and the pH range for Thymol Blue (base) is 8.0-9.6 (from the data booklet) which is WITHIN the range
 so giving a clear colour change
so giving a clear colour changeI'm sorry for making you write all that even after explaining.

Thank you for helping!

ethanoic acid is a weak acid (all carboxilic acids are weak) , naoh is a strong base.
If you drew/imagined a ph curve, it would start of at at around three, the acid is weak so its not very acidic, so the verticle region of the ph curve will start high (like 5-6) and since youre adding a STRONG base, the vertical region of the ph curve will be at a high ph (like till 12) then it curves off again, So the indicator you use should have a change in colour between the ph 7-11.
Thymol blue is 1.2-2.8 so cant use
Bromo is 6-7.6 too low aswell
Thymol is 8-9.6 so maybe
Azaril is 10-12.0 which seems alittle to high. You want the ph range to be as close to the middle of the verticle region as possible
This is what the ph curve would look like: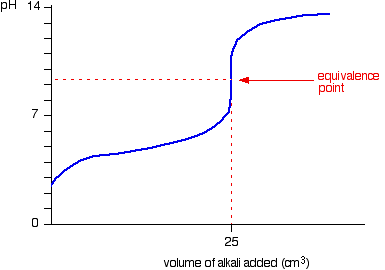
So thymol is the best bet.
Note:- once you know all your ph ranges, and the ph curves (how the curve looks like if its a weak or strong acid, and the same for a base) AND once you know what is a weak/strong acid/base. You should be able to do these easily.
If you drew/imagined a ph curve, it would start of at at around three, the acid is weak so its not very acidic, so the verticle region of the ph curve will start high (like 5-6) and since youre adding a STRONG base, the vertical region of the ph curve will be at a high ph (like till 12) then it curves off again, So the indicator you use should have a change in colour between the ph 7-11.
Thymol blue is 1.2-2.8 so cant use
Bromo is 6-7.6 too low aswell
Thymol is 8-9.6 so maybe
Azaril is 10-12.0 which seems alittle to high. You want the ph range to be as close to the middle of the verticle region as possible
This is what the ph curve would look like:

So thymol is the best bet.
Note:- once you know all your ph ranges, and the ph curves (how the curve looks like if its a weak or strong acid, and the same for a base) AND once you know what is a weak/strong acid/base. You should be able to do these easily.
Original post by ihaspotato
I'm sorry for making you write all that even after explaining. 
Thank you for helping!

Thank you for helping!

hahaha. you're welcome

Original post by AvWOW
For strong acids it's commonly Phenolpthalein
Where can I find the list to what is strong/weak acid and what is strong/weak base? or do I have to work that out myself?
(edited 7 years ago)
http://www.chemistry.pomona.edu/chemistry/1alab/www/fall2006/powerptpresentations/5anions/acidbaset.htm
http://www.science.uwaterloo.ca/~cchieh/cact/c123/wkacids.html
just remember the ones you usually come across
http://www.science.uwaterloo.ca/~cchieh/cact/c123/wkacids.html
just remember the ones you usually come across
Original post by Zahid~
http://www.chemistry.pomona.edu/chemistry/1alab/www/fall2006/powerptpresentations/5anions/acidbaset.htm
http://www.science.uwaterloo.ca/~cchieh/cact/c123/wkacids.html
just remember the ones you usually come across
http://www.science.uwaterloo.ca/~cchieh/cact/c123/wkacids.html
just remember the ones you usually come across
THANK YOU! I really needed this.
Don't know how to thank you more. Here's one from me.

(edited 7 years ago)
Original post by ihaspotato
Where can I find the list to what is strong/weak acid and what is strong/weak base? or do I have to work that out myself?
oops! I meant for strong BASES it's normally phenolpthalein :3 I didn't really have such a list, but weak acids tend to be things like carboxylic acids. You can compare the strength using the Ka values.
Strong tends to be more like H2SO4, HCl, HNO3 etc
Original post by AvWOW
oops! I meant for strong BASES it's normally phenolpthalein :3 I didn't really have such a list, but weak acids tend to be things like carboxylic acids. You can compare the strength using the Ka values.
Strong tends to be more like H2SO4, HCl, HNO3 etc
Strong tends to be more like H2SO4, HCl, HNO3 etc
Data booklet
could you explain how Ka values can be used to compare strong/weak acid bases? eg. larger Ka value stronger the acid?
Original post by ihaspotato
Data booklet
could you explain how Ka values can be used to compare strong/weak acid bases? eg. larger Ka value stronger the acid?
could you explain how Ka values can be used to compare strong/weak acid bases? eg. larger Ka value stronger the acid?
Found this. Posting it here if someone comes across this post asking the same question >>
https://www.khanacademy.org/science/organic-chemistry/organic-structures/acid-base-review/v/ka-and-acid-strength
still does not explain how to use Ka/pKa from the data booklet to for answering faster

(edited 7 years ago)
Original post by ihaspotato
Data booklet
could you explain how Ka values can be used to compare strong/weak acid bases? eg. larger Ka value stronger the acid?
could you explain how Ka values can be used to compare strong/weak acid bases? eg. larger Ka value stronger the acid?
I am soooo sorry but I just saw your message
 I really really hope you didn't suffer because I didn't check TSR
I really really hope you didn't suffer because I didn't check TSR 
Again so so sorry. Hope you found the paper well? IAL or GCE?
Original post by AvWOW
I am soooo sorry but I just saw your message  I really really hope you didn't suffer because I didn't check TSR
I really really hope you didn't suffer because I didn't check TSR 
Again so so sorry. Hope you found the paper well? IAL or GCE?
 I really really hope you didn't suffer because I didn't check TSR
I really really hope you didn't suffer because I didn't check TSR 
Again so so sorry. Hope you found the paper well? IAL or GCE?
No worries mate! I learn't it from a friend right before I entered the exam venue.

The paper (IAL) went kinda well... wouldn't say it was my best paper or the worst that I gave. Plus I don't even remember what the questions were or the answers that I gave - after I left the exam hall. I went to the chem discussion here and the replies on that thread are kind of scary since I wish I knew what they were talking about - making me feel like I gave all the wrong answers.

But it's no use worrying about it now... Unit 5 Chem has got to go good no matter what!
(edited 7 years ago)
Original post by ihaspotato
No worries mate! I learn't it from a friend right before I entered the exam venue. 
The paper (IAL) went kinda well... wouldn't say it was my best paper or the worst that I gave. Plus I don't even remember what the questions were or the answers that I gave - after I left the exam hall. I went to the chem discussion here and the replies on that thread are kind of scary since I wish I knew what they were talking about - making me feel like I gave all the wrong answers.
But it's no use worrying about it now... Unit 5 Chem has got to go good no matter what!

The paper (IAL) went kinda well... wouldn't say it was my best paper or the worst that I gave. Plus I don't even remember what the questions were or the answers that I gave - after I left the exam hall. I went to the chem discussion here and the replies on that thread are kind of scary since I wish I knew what they were talking about - making me feel like I gave all the wrong answers.

But it's no use worrying about it now... Unit 5 Chem has got to go good no matter what!
IAL too! I found it to be the easiest paper so far... Which makes me just a little afraid of unit 5.
But with a unit 6 like that, they really can't make it any harder
Quick Reply
Related discussions
- AQA A Level Chemistry Paper 3 20th June 2018 Unofficial Markscheme
- Indicator colour question.
- A level chemistry
- Oxford AQA iGCSE Chemistry
- IAL Chemistry - Unit 4 EXAM DISCUSSION
- AQA A Level chemistry tests
- Applied Science Unit 3 investigation skills
- Kc Titration Question
- reuben's y13/medapps journey!!
- Biology A level maths question
- 25 marker essay biology
- As level / a level chemistry concentration help!!
- A-level Chemistry Study Group 2022-2023
- Aluminium oxide amphoteric
- Edexcel GCSE Biology Paper 1 Higher Tier [16th May 2023] Exam Chat
- DNA multiple choice question
- urgent chemistry question!! a level
- BTEC applied science unit 19
- Biology and chemistry
- A level revision songs
Latest
Last reply 13 minutes ago
Official University of St Andrews Applicant Thread for 2024Last reply 24 minutes ago
Standard Chartered Apprenticeships 2024Last reply 24 minutes ago
Children to no longer be prescribed puberty blockers, NHS England confirmsLast reply 26 minutes ago
Why is the political left now censorious and authoritarian??Last reply 27 minutes ago
narrowly missing offer conditionsLast reply 32 minutes ago
St dominics, henrietta barnett, RMS, parmiters or girls grammar??Last reply 33 minutes ago
2024 entry A100 / A101 Medicine fastest and slowest offer sendersMedicine
857
Last reply 35 minutes ago
Medical doctor degree apprenticeship 2024Last reply 37 minutes ago
yet to receive a woodhouse interviewLast reply 37 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 39 minutes ago
What are your thoughts on poverty eradication programs in the worldLast reply 46 minutes ago
UAL Bsc Fashion Management and fashion marketingLast reply 47 minutes ago
Choosing Subjects for S5 to study Software engineering in the Uk



