Edexcel A2 Chemistry Exams -6CH04 (14th June) and 6CH05 (22nd June) Discussion Thread
Scroll to see replies
That was a very nice paper.
Bit of a trick question about the observation when the thiosulfate ions are used up - quite a few people I know said blue/black to colourless (wrong way around I believe).
Bit of a trick question about the observation when the thiosulfate ions are used up - quite a few people I know said blue/black to colourless (wrong way around I believe).
Original post by lfcrules
Would it be ok if I said the strsch turns blue black , nothing about colourless to blue black
I think so, it was only one mark if i think
Original post by lfcrules
Would it be ok if I said the strsch turns blue black , nothing about colourless to blue black
Should alright hopefully, Edexcel aren't too harsh in Mark schemes. That's what I put anyway
 .
.Nonetheless a pretty straightforward paper despite having a couple of oddball questions. Hope everyone found it alright! Unit 5 to go!!
This is the pH Curve roughly i believe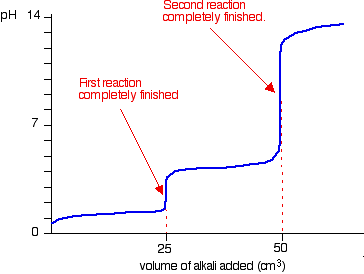

What were the three differences you guys put for acid/alkali hydrolysis. And what 2 bonds did you say we're different for the last bit on the nmr question
Posted from TSR Mobile
Posted from TSR Mobile
Original post by CLGC98
What was the answer for thr final question??? Abiut ir spectrum??
It was either absence/presence of C-Cl, C-O, H-O or value for c=o being different
Original post by C0balt
It was either absence/presence of C-Cl, C-O, H-O or value for c=o being different
I talked about it in terms of COCl and COOH groups. Is that alright do you think because in the question it said wavenumbers for relevant bonds or groups involved?
What wavenumbers did you use btw?
Original post by WashingPowder
This is the pH Curve roughly i believe

Yeah I did this as well but I believe the graph should start from 1.5 as that was the ph of H2SO4
(edited 7 years ago)
Original post by C0balt
It was either absence/presence of C-Cl, C-O, H-O or value for c=o being different
I said there would be OH for the phenol group in both but for the carboxylic acid group AS well there would be a different OH bond absorption as well?
Original post by C0balt
It was either absence/presence of C-Cl, C-O, H-O or value for c=o being different
I just said salicylic acid had a sharp peak at he wavenumber for C=O in carboxylic acid ( dont remember the wavenumber)
Amd said had a peak for the acyl chloride group
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- Edexcel GCSE Physics Paper 2 Higher (1PH0/2H) - 14th June 2024 [Exam Chat]
- Edexcel GCSE Physics Paper 2 Foundation (1PH0/2F) - 14th June 2024 [Exam Chat]
- Edexcel GCSE Combined Science Paper 6 Physics 2 Foundation- 14th Jun 2024 [Exam Chat]
- Edexcel GCSE Geography A Paper 3 (1GA0/03) - 14th June 2024 [Exam Chat]
- Edexcel GCSE Combined Science Paper 6 Physics 2 Higher - 14th June 2024 [Exam Chat]
- Edexcel A-level Biology A (Salters-Nuffield) Paper 2 - 14th June 2024 [Exam Chat]
- Edexcel A Level Politics Paper 3 (9PL0 3A/3B) - 14th June 2024 [Exam Chat]
- Go ahead in time as far as possible
- Edexcel A-level Spanish Paper 2 (9SP0 02) - 14th June 2023 [Exam Chat]
- Edexcel GCSE Combined Sci Paper 2 Foundation (1SC0 2CF) - 13th June 2023 [Exam Chat]
- AQA GCSE Chemistry Paper 2 Foundation Tier (8462 2F) - 13 June 2023 [Exam Chat]
- Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]
- Edexcel GCSE Combined Science Paper 5 Chem 2 Higher - 11th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 3 (8035/3) - 14th June 2024 [Exam Chat]
- Edexcel GCSE Foundation tier Maths Paper 3 3F (1MA1) - 14th June 2023 [Exam Chat]
- Edexcel A-level English Literature Paper 3 (9ET0 3) - 14th June 2024 [Exam Chat]
Latest
Last reply 1 minute ago
Official University of St Andrews Applicant Thread for 2024Last reply 10 minutes ago
Official University College London Applicant Thread for 2024Last reply 10 minutes ago
Official University of Bath Offer Holders Thread for 2024 entryLast reply 11 minutes ago
Michaela School: Muslim student loses prayer ban challengeLast reply 13 minutes ago
Civil service - location allocations and preference DWPLast reply 17 minutes ago
International graduate student looking for graduate scheme that sponsors visa.Last reply 19 minutes ago
Can someone please mark this out of 30(all my teachers refused)Last reply 20 minutes ago
LSE Undergraduate Politics and IR 2024Last reply 21 minutes ago
Will an IVA cause my Civil Service pre employment check to fail?Last reply 21 minutes ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadTrending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]


