Aqa chem 4/ chem 5 june 2016 thread
Scroll to see replies
Original post by Parallex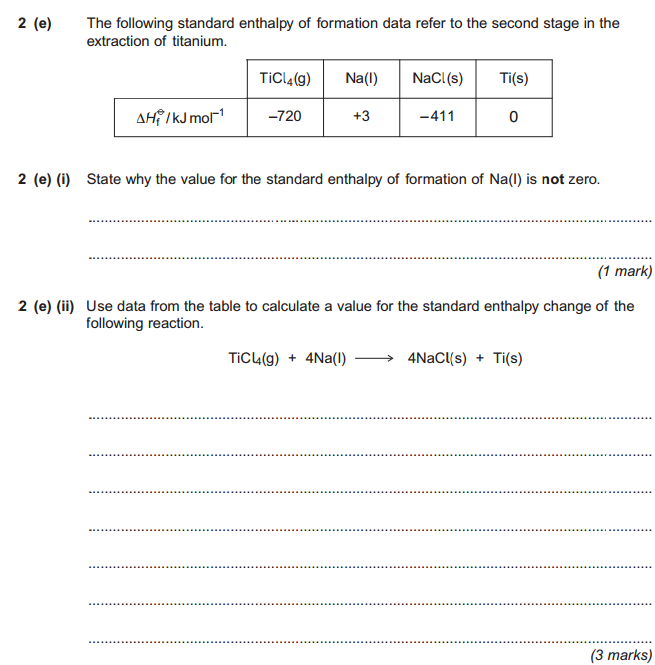
@Aerosmith
Explain to me how this is any different to the question. You're provided with enthalpy of formation values and asked to work out the standard enthalpy change of the following reaction exactly as we did.
Just telling you now, there's absolutely no diving in the mark scheme.

@Aerosmith
Explain to me how this is any different to the question. You're provided with enthalpy of formation values and asked to work out the standard enthalpy change of the following reaction exactly as we did.
Just telling you now, there's absolutely no diving in the mark scheme.
Na isn't in its standard state so formation of 1 mol would not be required. Do you now see the logic?
I put increasing SA would increase emf 
ALL SETTLED- You did not need to divide by 2.
THE END of that

ALL SETTLED- You did not need to divide by 2.
THE END of that
Original post by Darthsion100
I got something like 2328 KJ Mol-1 at first, then I halved the atomisation of oxygen to get 2404, but I think the original answer of 2328 was correct, because atomisation is for one mole, and you got 1 mole of O
oh **** yeah ur right, ah well should only be one mark right?
Original post by Suits101
I got that quote from the internet... The top thing that comes up when when I googled standard enthalpy change? Lol.
Well I put kJ/mol so it should be right if what you say is right regardless.
Well I put kJ/mol so it should be right if what you say is right regardless.

No it wont be right, because you have divided your enthalpy by 2 and I presume your entropy by 2 - you can expect to drop 2/3 marks.
Next time don't take to TSR trying to tell the 95% of people who didn't get your answer that they are wrong, causing unnecessary stress for people.
Was Gibbs free worth 3 marks?
Also can anyone confirm for MgCl2 solubility one:
Solubility decreases
Forward reaction is exothermic
Equilibrium shifts left to oppose increase in temperature
Also can anyone confirm for MgCl2 solubility one:
Solubility decreases
Forward reaction is exothermic
Equilibrium shifts left to oppose increase in temperature
Original post by Aerosmith
Na isn't in its standard state so formation of 1 mol would not be required. Do you now see the logic?
No.
how many marks do you lose for not converting degrees to kelvin?
Original post by Parallex
No.
I think you're just a stubborn nerd who can't accept that it was perfectly fine to divide by 2 and work it out
Original post by j5994
oh **** yeah ur right, ah well should only be one mark right?
Lets both just prey :/
Original post by Suits101
It came up!!! 



wtf is the chelate effect?
Original post by Aerosmith
Na isn't in its standard state so formation of 1 mol would not be required. Do you now see the logic?
I'm sorry, with all due respect aerosmith, that comment (plus perhaps most/all of your previous comments you've written in this discussion) is just absolute nonsense.
Na isn't in its standard state? Ok... isn't that why it's delta H value isn't 0 then?
I'm sorry, to be honest I've got no idea what 'logic' you refer to, but long story short Parallex has used an absolutely valid reference to a question and there's nothing that prevents comparison of that question with the one in our exam
Original post by Suits101
Was Gibbs free worth 3 marks?
Also can anyone confirm for MgCl2 solubility one:
Solubility decreases
Forward reaction is exothermic
Equilibrium shifts left to oppose increase in temperature
Also can anyone confirm for MgCl2 solubility one:
Solubility decreases
Forward reaction is exothermic
Equilibrium shifts left to oppose increase in temperature
Solubility one- AGREED

Original post by Aerosmith
I think you're just a stubborn nerd who can't accept that it was perfectly fine to divide by 2 and work it out
So right I can't wait for people to see the paper again because it asked for delta Hf
Original post by Suits101
Was Gibbs free worth 3 marks?
Also can anyone confirm for MgCl2 solubility one:
Solubility decreases
Forward reaction is exothermic
Equilibrium shifts left to oppose increase in temperature
Also can anyone confirm for MgCl2 solubility one:
Solubility decreases
Forward reaction is exothermic
Equilibrium shifts left to oppose increase in temperature
YES! I got this, but people were telling me it's wrong. I think I've dropped 3 marks in total, I don't want a CE and to make it 6!!
Original post by RME11
No it wont be right, because you have divided your enthalpy by 2 and I presume your entropy by 2 - you can expect to drop 2/3 marks.
Next time don't take to TSR trying to tell the 95% of people who didn't get your answer that they are wrong, causing unnecessary stress for people.
Next time don't take to TSR trying to tell the 95% of people who didn't get your answer that they are wrong, causing unnecessary stress for people.
Drop 2 marks if its wrong.
I always write what I have done and I said I BELIEVE that's what you do unless I am certain, you can check my posts.
I have never said that's the proper way to do it because of that reason, and I don't want to argue with you (especially because I have a final exam tomorrow and I don't see the need).
Jesus people, it's chemistry not game of enthalpy changes
Do you divide by 2 or not this is doing my head in now
Original post by Spectral
I'm sorry, with all due respect aerosmith, that comment (plus perhaps most/all of your previous comments you've written in this discussion) is just absolute nonsense.
Na isn't in its standard state? Ok... isn't that why it's delta H value isn't 0 then?
I'm sorry, to be honest I've got no idea what 'logic' you refer to, but long story short Parallex has used an absolutely valid reference to a question and there's nothing that prevents comparison of that question with the one in our exam
Na isn't in its standard state? Ok... isn't that why it's delta H value isn't 0 then?
I'm sorry, to be honest I've got no idea what 'logic' you refer to, but long story short Parallex has used an absolutely valid reference to a question and there's nothing that prevents comparison of that question with the one in our exam
To be honest with you, I think it's poor wording on AQA's part and so both should be accepted
For the dividing by 2, I was looking at the question in the exam trying to figure out whether it was necessary or not! I think that if answers are given as for example: -189 kj for the enthalpy change for the reaction then that's fine, and if given as -94.5 kj mol-1 that's also fine. Both are the same it's just the units that change and I think if you changed units depending on your answer you should get the marks. Agree?
Original post by Aethrell
oh for ****s sake, completely forgot
Are we sure it said under standard conditions?
Quick Reply
Related discussions
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- GCSE Exam Discussions 2024
- TSR Study Together - STEM vs Humanities!
- A-level Exam Discussions 2024
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- reuben's y13/medapps journey!!
- A-level Biology Study Group 2022-2023
- Need Jan 2022 Past papers - Oxford AQA international A level BL05
- Grade Growth Chronicles | From C's to A's (23-24)
- Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05
- better late than never - gyg 24' :)
- GCSE Results: Post your results
- Holding myself accountable; study!
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
- A Level Exam Discussions 2023
- C in Biology and D in Chem, while I resit could I do an intensive alevel in maths
- GYG a level y13⋆୨୧˚⟡˖ ࣪
- AS chemistry paper 2 2022 AQ
- AQA A Level Chemistry Paper 1 Practicals
- Biology Paper 1 - PRACTICE exam paper (with mark scheme) INVALUABLE resource
Latest
Last reply 1 minute ago
AQA A Level Sociology Paper 2 (7192/2) - 4th June 2024 [Exam Chat]Last reply 2 minutes ago
R u 18-25 & studying in a England Uni, spare a few mins to fill my survey. 1 wk left.Last reply 5 minutes ago
Edexcel A Level Economics A Paper 1 (9ECO 01) - 15th May 2024 [Exam Chat]Posted 12 minutes ago
st. andrews sixth form in cambridgeLast reply 30 minutes ago
Anyone else applying/has applied to UCL's Robotics and AI MEng course??Last reply 43 minutes ago
LSE International Social and Public Policy and Economics (LLK1) 2024 ThreadLast reply 1 hour ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadLast reply 1 hour ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 hour ago
Official University of the Arts London Applicant Thread for 2024Last reply 1 hour ago
LAWYERS: Can I bring an action against my old schoolLast reply 1 hour ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 1 hour ago
Children to no longer be prescribed puberty blockers, NHS England confirmsLast reply 2 hours ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1275
Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



