Chemistry help please
What does the percentage abundance mean? How does a mass spectrometer work? Someone please help me, I dont understand this
I just need to understand it, please help me
Thankyou so much, I understand it now x 

I can't help you loads. If could have helped if you had more specific questions in mind, but regardless...

Do you know why chlorine has the atomic mass of 35.5?
Well the percentage abundance is just the abundance of the isotope as a percentage. Chlorine is found naturally in the atmosphere in two isotopes (there may be more but they're so small that we don't count them). 75% of chlorine naturally found is of the isotope Cl-35.
That means 0.75*35 = 26.25
0.25*37 = 9.25
Add those together...35.5.
As for mass spectrometry, you don't need to heavily know how this works...
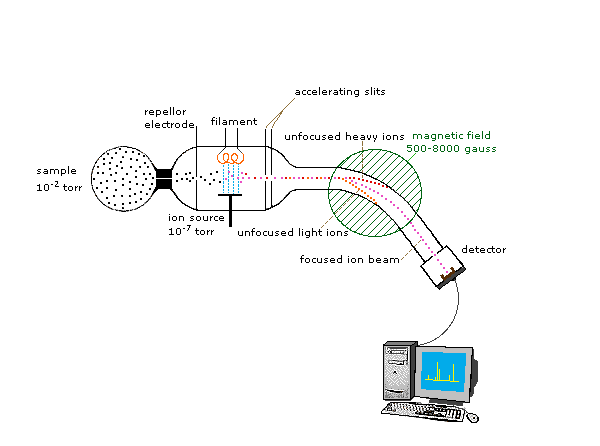
Just read these... Then read your textbook and answer the questions.
http://www.chemguide.co.uk/analysis/masspecmenu.html#top
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/massspec/masspec1.htm

Do you know why chlorine has the atomic mass of 35.5?
Well the percentage abundance is just the abundance of the isotope as a percentage. Chlorine is found naturally in the atmosphere in two isotopes (there may be more but they're so small that we don't count them). 75% of chlorine naturally found is of the isotope Cl-35.
That means 0.75*35 = 26.25
0.25*37 = 9.25
Add those together...35.5.
As for mass spectrometry, you don't need to heavily know how this works...

Just read these... Then read your textbook and answer the questions.
http://www.chemguide.co.uk/analysis/masspecmenu.html#top
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/massspec/masspec1.htm
Quick Reply
Related discussions
- Bangor University GCSE Revision guides?
- TSR Study Together - STEM vs Humanities!
- A level 2023 exams
- ‼️OCR Science URGENT help needed‼️‼️‼️‼️‼️
- Worried about studying Chemistry at UNI
- Physiotherapy or pharmacy
- PHARMACY INTERVIEW @ Portsmouth
- Oxford Chemistry Interview
- Oxford Chemistry Interview
- Struggling in A level chemistry
- I want to change my Major.
- I need to improve my chemistry grades; I want to do medicine
- Biomed vs Biochem vs chemistry
- Mnemonics for the Reactivity Series
- Worried about degree difficulty - Chemistry
- Official University of Lincoln 2023 Applicant Thread
- Specific Charge
- Pharmacy at UCL GCSE grades entry requirements 2021
- Chemistry revision
- Chemistry degree
Latest
Last reply 29 minutes ago
Got my crush's number 2 days ago and no reply yet. What could be the reasons?Posted 33 minutes ago
GB News set to axe 40 jobs after channel posts heavy lossesPosted 40 minutes ago
Sunak rejects offer of youth mobility scheme between EU and UKLast reply 1 hour ago
Bank Of england degree apprenticeship 2024Last reply 1 hour ago
Feeling inferior compared to syrians as a half moroccan/half whiteLast reply 1 hour ago
LSE International Social and Public Policy and Economics (LLK1) 2024 ThreadLast reply 1 hour ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 hour ago
Atkins Degree Apprenticeship 2024Last reply 1 hour ago
Official University of St Andrews Applicant Thread for 2024



