This discussion is now closed.
Check out other Related discussions
- Does sixth form school matter?
- Bad A level combination
- Advice on choosing degree??!?
- A-level/Career Choice Advice
- University Choice
- computer science- worth it?
- I dunno what subject to drop
- Oxford University for Computer Science
- Which university for Computer science
- royal holloway biomedical science or durham biological sciences???
- computer science at university
- Gcse Options Year 9
- failed History
- Physics and Maths tutor
- how vital is comp sci for a comp sci uni course
- A level 3rd Subject
- How do stand out when applying for a Masters? Comp sci to be specific.
- Triple Science vs Combined Higher
- Physics at oxbridge
- Do you think it should be important to pass GCSE science to go onto A Levels?
Which do you think is the most important science?
Scroll to see replies
Biology all the way! 

Original post by Absent Agent
Yes, philosophy is not considered a science in the everyday sense of the word, but a theoretical science. But do you know why the word PhD stands for the Doctorate of Philosophy?
.........which came from Western tradition (which traditionally emphasised humanities and religion over science), and not by logical design.
In the same way Philosophy only really touched upon ideas in Europe, largely ignoring the rest of the world... which leads me to address your next comment:
Original post by Absent Agent
That's a bit of mischaracterising the subject-matter of philosophy to be honest. You are taught those literacy skills in order to get your point across effectively. Although many people would define philosophy differently, its subject-matter does involve studying the foundation of knowledge.
It's not the foundation of knowledge unless you qualify that with 'in Europe' or 'in the western world'. It is largely a western-centric study of mostly western knowledge. To say it is the foundation of knowledge is at best a misconception, at worst a blatant lie.
I wonder what would Philosophy be, if it did include ideas from the whole world? Probably, the Greek/Roman/European philosophers would only be a very small part of it. But it would then it might go towards the claim to be the foundation of knowledge.
Original post by ThePricklyOne
The highlighted areas were created and developed by mathematicians* working for spy agencies and the military, not physicists. If you meant nuclear and rocket technology, now that was developed by a bunch of physicists (who were awesome!).
These same mathematicians then created the concept of electronic databases to run on these huge computers using relational algebra and calculus. You punch in the code telling the machine to fetch/ shape / slice your data, the machines performs the maths to go and do it for you. Telecommunication worked in a similar fashion - data was encapsulated in code split into chunks, sent over the wire and then reassembled into my mom's Christmas message using mathematics.
* these mathematicians then tried to solve a problem created by the physicists - nuclear war. In the event the physicists' invention the nuclear bomb destroyed everything, there needed to be something to allow the remnants of mankind and the military in their secret bunkers to communicate so they can rebuild society. Thus these mathematicians invented the most time wasting invention known to man - the Internet.
.......................followed by cute cat videos......
These same mathematicians then created the concept of electronic databases to run on these huge computers using relational algebra and calculus. You punch in the code telling the machine to fetch/ shape / slice your data, the machines performs the maths to go and do it for you. Telecommunication worked in a similar fashion - data was encapsulated in code split into chunks, sent over the wire and then reassembled into my mom's Christmas message using mathematics.
* these mathematicians then tried to solve a problem created by the physicists - nuclear war. In the event the physicists' invention the nuclear bomb destroyed everything, there needed to be something to allow the remnants of mankind and the military in their secret bunkers to communicate so they can rebuild society. Thus these mathematicians invented the most time wasting invention known to man - the Internet.
.......................followed by cute cat videos......
Also, telecommunications was not developed by mathematicians - unless you're counting Maxwell, who did nothing more than theorize that light and electromagnetic waves were the same thing and could be propagated through free space. The people who used those equations to develop wireless telegraphy were physicists (Hughes, Hertz, Marconi, et al).
You claim that nuclear and rocket technologies were developed by physicists. Maybe that's true - but they were conceived by mathematicians too (most notably nuclear, which was famously conceived - and later regretted - by Albert Einstein).
Now, ask yourself two questions:
1.
Did Tootles reply to the thread that OP posted, in the spirit that the OP originally (apparently) intended? and
2.
am I (are you) being a clever Dick?
Original post by Tootles
The OP gave a choice of physics, chemistry, or biology, so I chose physics. Also I wasn't talking of the founding principles of computer science - I probably know at least as much about those as you - I was talking about the implementations of those on which we have relied for so many decades, which were developed using principles developed by physicists.
Also, telecommunications was not developed by mathematicians - unless you're counting Maxwell, who did nothing more than theorize that light and electromagnetic waves were the same thing and could be propagated through free space. The people who used those equations to develop wireless telegraphy were physicists (Hughes, Hertz, Marconi, et al).
You claim that nuclear and rocket technologies were developed by physicists. Maybe that's true - but they were conceived by mathematicians too (most notably nuclear, which was famously conceived - and later regretted - by Albert Einstein).
Now, ask yourself two questions:
Also, telecommunications was not developed by mathematicians - unless you're counting Maxwell, who did nothing more than theorize that light and electromagnetic waves were the same thing and could be propagated through free space. The people who used those equations to develop wireless telegraphy were physicists (Hughes, Hertz, Marconi, et al).
You claim that nuclear and rocket technologies were developed by physicists. Maybe that's true - but they were conceived by mathematicians too (most notably nuclear, which was famously conceived - and later regretted - by Albert Einstein).
Now, ask yourself two questions:
1.
Did Tootles reply to the thread that OP posted, in the spirit that the OP originally (apparently) intended? and
2.
am I (are you) being a clever Dick?
Albert Einstein is a physicist. you are most welcome to disagree.
I like an argument as good as anybody but.........
In response to your inappropriately aggressive manner..
1) This is a public forum. If you do not want others to participate, please PM the OP or mark your response to OP only (i.e. you don't want anyone else to reply) or start a thread stating you want only certain people to join.
2) if you do not like the post - you have the option of ignoring it & the discussion will stop there. See? Easy.
2) Please do not pass an insult as a question. It is still an insult. So grow up.

Original post by Plantagenet Crown
It's the chemical properties of elements. Chemistry is the study of atoms, so electrons, protons etc are still part of chemistry, the subject doesn't just stop at molecules. And once again, you don't need an advanced understanding of quantum mechanics to be able to predict and carry out reactions.
The chemical properties of elements result from the physical properties of them. For example, Flourine is a toxic gas, and we both can agree that it's a chemical property of it. Surely it's toxic because it's an oxidsing agent, a powerful one in fact. But this oxidising power emerge from the electrostatic force of the nucleus of fluorine, and its small atomic radius adds to that effect. As a result, this oxiding power in turn give rise to other chemical properties of a wider class of compounds in which flourine can be found.
Original post by Plantagenet Crown
Organic chemists had no problem doing reactions before quantum mechanics was even discovered, how do you explain that?
And you don't need to know about physical chemistry to predict the outcomes of reactions.
And you don't need to know about physical chemistry to predict the outcomes of reactions.
That's correct! As I said before, the theoretical approach is just a bonus accredited to quantum mechanics. Experimental chemists of old times had to carry out numerous reactions to arrive at new hypotheses, but nowadays, you are already equipped with those hypotheses and know what to expect from carrying out a set of reactions.
Original post by Plantagenet Crown
And no, you can't always skip the practical approach, because reactions don't always happen the way you think they will. I don't think you would be saying this if you had ever actually been in a lab and done chemical reactions...
As I said, I'm doing a PhD in organic chemistry and I have no problem predicting and analysing reactions despite not having done quantum mechanics since my second year and remembering basically nothing about it..
As I said, I'm doing a PhD in organic chemistry and I have no problem predicting and analysing reactions despite not having done quantum mechanics since my second year and remembering basically nothing about it..
Yes, and I guess what I said was quite a bold claim, but what obvious I meant was that, as I pointed this before, it would be quantum mechanics that would provide explanations for these anomalies in chemical reactions.
Original post by Plantagenet Crown
Silly point to make as then we'd get into the topic of engineering. How nuclear magnetic resonance actually works is dependent on the property of atoms, which is chemistry.
When I said how the the devices are made, I meant the knowledge that were required in building them. Surely the context in which I said that proves this. Anyway, A quick browse through the internet shows that the inventors of NMR were physicist who received the 1952 Nobel Prize in Physics.
Original post by Plantagenet Crown
There is a lot of overlap between chemistry of course, but to say quantum mechanics is necessary to work in all areas of chemistry is just absurd. The overwhelming majority of chemists who don't work in the field of quantum mechanics are obvious proof that what you're saying isn't true.
Again, I never implied that, and of course that would be very bold claim, but I do say that it is quantum mechanics that provides the rich understanding of chemical behavior.
(edited 7 years ago)
Original post by Plantagenet Crown
I don't think this is a very useful question because the different sciences deal with different areas. Physics will be useless when dealing with organisms, medicine etc and biology will be useless when talking about how natural laws work. Chemistry is the central science, but there are also many areas where biology or physics will be more useful so it really depends on the scientific context.
And this is coming from a chemist.
And this is coming from a chemist.
Physicist in complete agreement

Important from an academic standpoint? -It depends on your intended career path. There is no 'better' as it's all a matter of opinion and any attempt to categorise will be subject to bias.
Original post by ThePricklyOne
.........which came from Western tradition (which traditionally emphasised humanities and religion over science), and not by logical design.
In the same way Philosophy only really touched upon ideas in Europe, largely ignoring the rest of the world... which leads me to address your next comment.
In the same way Philosophy only really touched upon ideas in Europe, largely ignoring the rest of the world... which leads me to address your next comment.
Philosophy has always dealt with different enquiries, which in turn led to the emergence of different branches of philosophy, with epistemology and/metaphysics only responsible for the foundation of knowledge.
Original post by ThePricklyOne
It's not the foundation of knowledge unless you qualify that with 'in Europe' or 'in the western world'. It is largely a western-centric study of mostly western knowledge. To say it is the foundation of knowledge is at best a misconception, at worst a blatant lie.
I wonder what would Philosophy be, if it did include ideas from the whole world? Probably, the Greek/Roman/European philosophers would only be a very small part of it. But it would then it might go towards the claim to be the foundation of knowledge.
I wonder what would Philosophy be, if it did include ideas from the whole world? Probably, the Greek/Roman/European philosophers would only be a very small part of it. But it would then it might go towards the claim to be the foundation of knowledge.
I don't quite understand what you mean there, but different countries had little, if any, to do what philosophy was about. The only difference was that over different periods, and around different parts of the world, the focus of philosophy was shifted to different areas of inquiries. For example, Romans mostly dealt with moral philosophy, while the medieval philosophy was highly theological. In the someway, the twentieth-century's had its focus on philosophy of language and logic, but philosophy remained what it was.
Original post by The Joker ~
"Rocket science" we'll be needing a new planet soon ~
Maybe biology and understanding of environmental sciences (biol, chem and physics) so we don't need them rockets and can take care of the planet we currently call home instead
Original post by Absent Agent
The chemical properties of elements result from the physical properties of them. For example, Flourine is a toxic gas, and we both can agree that it's a chemical property of it. Surely it's toxic because it's an oxidsing agent, a powerful one in fact. But this oxidising power emerge from the electrostatic force of the nucleus of fluorine, and its small atomic radius adds to that effect. As a result, this oxiding power in turn give rise to other chemical properties of a wider class of compounds in which flourine can be found.
Chemical properties' link to physical properties is still chemistry! Protons, electrons, neutrons are all studied in chemistry and part of it. Again, chemistry doesn't just stop at the molecular level, it also studies atoms themselves and their constituent parts.
That's correct! As I said before, the theoretical approach is just a bonus accredited to quantum mechanics. Experimental chemists of old times had to carry out numerous reactions to arrive at new hypotheses, but nowadays, you are already equipped with those hypotheses and know what to expect from carrying out a set of reactions.
Again, this is absolutely false. The vast majority of reactions can be predicted fairly accurately without having the slightest understanding of quantum mechanics. All you really need to do is the chemical properties of elements and functional groups.
Yes, and I guess what I said was quite a bold claim, but what obvious I meant was that, as I pointed this before, it would be quantum mechanics that would provide explanations for these anomalies in chemical reactions.
You don't need quantum mechanics to explain it, it can be explained in organic chemistry terms. Again, you will find very few organic papers and journals that justify their reactions using physical chemistry.
You were saying a knowledge of quantum mechanics is necessary to do chemistry and this is categorically false. The overwhelming majority of chemists don't work in the field of quantum mechanics and will therefore know very little, if anything about it. And they still manage to do chemistry effectively.
When I said how the the devices are made, I meant the knowledge that were required in building them. Surely the context in which I said that proves this. Anyway, A quick browse through the internet shows that the inventors of NMR were physicist who received the 1952 Nobel Prize in Physics.
It is irrelevant who invented NMR as the concept itself relies on the properties of molecules and atoms, which is undeniably chemistry. The knowledge required to build actual machines would be engineering and a whole host of other fields. If you want to go down that pedantic route then chemistry would also be involved in making the machines, as you'd need to know the properties of materials.
Again, I never implied that, and of course that would be very bold claim, but I do say that it is quantum mechanics that provides the rich understanding of chemical behavior.
(edited 7 years ago)
Original post by Plantagenet Crown
Chemical properties' link to physical properties is still chemistry! Protons, electrons, neutrons are all studied in chemistry and part of it. Again, chemistry doesn't just stop at the molecular level, it also studies atoms themselves and their constituent parts.
Of course there should be a boundary so that these different fields can be classified, but, as I said, those properties are essentially the knowledge of physics, and if you study them in the field of chemistry, it's simply because you need them.
Original post by Plantagenet Crown
Again, this is absolutely false. The vast majority of reactions can be predicted fairly accurately without having the slightest understanding of quantum mechanics. All you really need to do is the chemical properties of elements and functional groups. You don't need quantum mechanics to explain it, it can be explained in organic chemistry terms. Again, you will find very few organic papers and journals that justify their reactions using physical chemistry.
I've said this before, but even the knowledge of functional groups or elements are just ready-made from the physicist handed over to the chemist. Here is an organic-chemistry data sheet from the web:
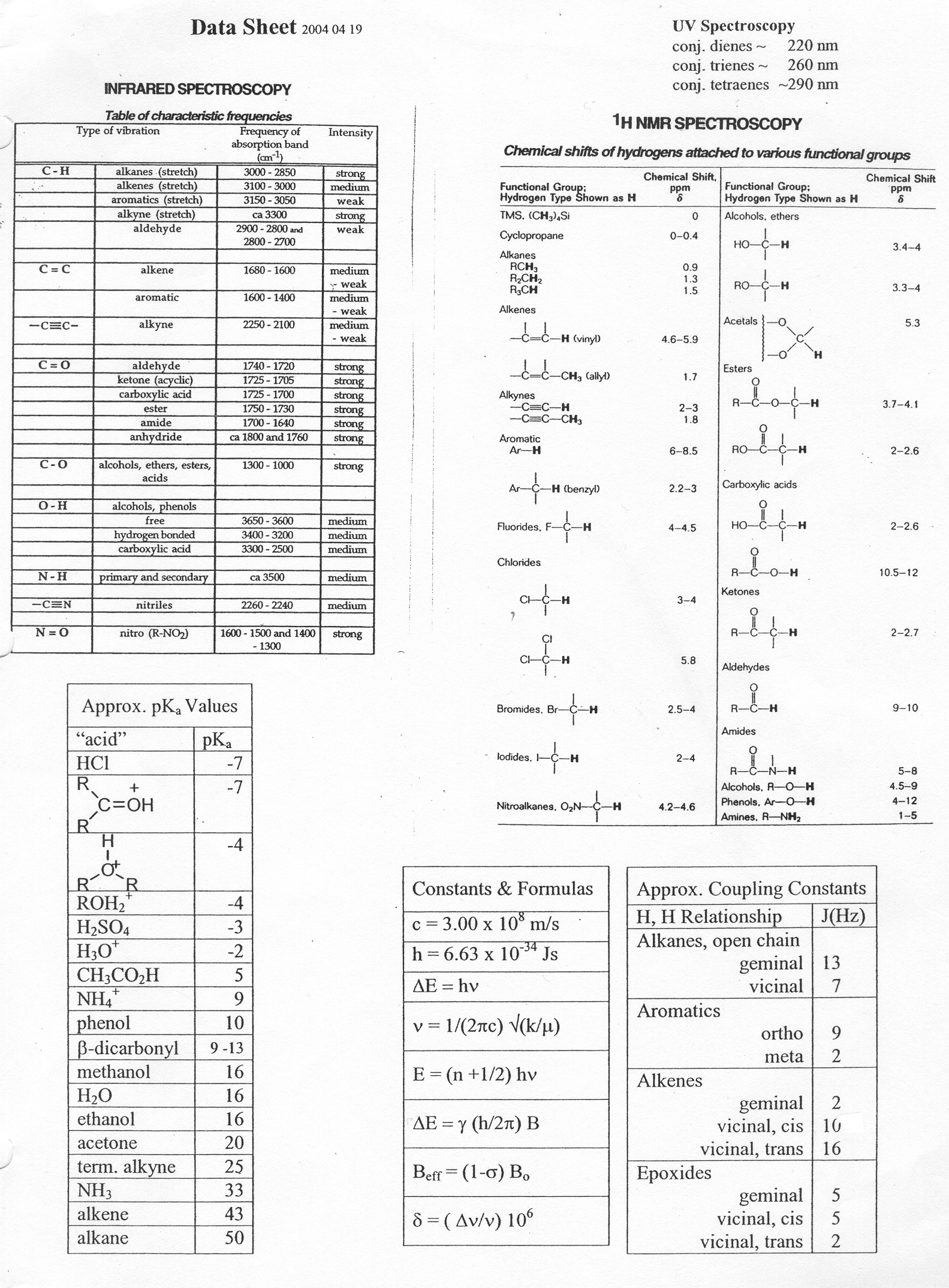
I can hardly understand why these data are not ready-made from the physicist.
Original post by Plantagenet Crown
You were saying a knowledge of quantum mechanics is necessary to do chemistry and this is categorically false. The overwhelming majority of chemists don't work in the field of quantum mechanics and will therefore know very little, if anything about it. And they still manage to do chemistry.
I never said that it was necessary. Let me quote my first post (although I changed the wording a bit so that it makes sense better) on the thread to which you first replied:
The properties of different materials, the whole Chemistry, has become for the first time the objects that could be predicted from the theory, and not from hypotheses deduced from experiments, which could not have been possible without a deep command of Quantum Mechanics.
These lines summarise what I've been saying, and you can see that I did not imply that knowledge of quantum mechanics was necessary to do chemistry, and I have said that a few times now. What I'm saying is that quantum mechanics has been able to predict the outcomes of reactions and many other chemical properties purely from the knowledge and behavior of subatomic particles.
Original post by Plantagenet Crown
It is irrelevant who invented NMR as the concept itself relies on the properties of molecules and atoms, which is undeniably chemistry. The knowledge required to build actual machines would be engineering and a whole host of other fields. If you want to go down that pedantic route then chemistry would also be involved in making the machines, as you'd need to know the properties of materials.
Well, engineering just seeks applications of scientific knowledge, and, in the case of inventing the NMR machine, the required knowledge is from physics. But I'm going to leave this discussion and let the audience to decide whether the foundation of chemistry, or even organic chemistry, is built upon physics.
Original post by Alex_Rasdra
Maybe biology and understanding of environmental sciences (biol, chem and physics) so we don't need them rockets and can take care of the planet we currently call home instead
My dear Alex ~ telling the commoner about the importance of preserving the planet is one thing.. But having leaders who deny the very existence of global warming and in a broader sense of protecting environment, simply because the place where he stands is not any warmer than yesterday or some years ago is another!
Rocket science ... the sooner the better ~
Original post by The Joker ~
My dear Alex ~ telling the commoner about the importance of preserving the planet is one thing.. But having leaders who deny the very existence of global warming and in a broader sense of protecting environment, simply because the place where he stands is not any warmer than yesterday or some years ago is another!
Rocket science ... the sooner the better ~
Rocket science ... the sooner the better ~
~wishes the star trek federation political idea was a thing IRL ... space travel, peaceful societies without war and replicators making all my food

edit... minus the borg maybe
I personally think that the most important is Chemistry because it helps us gain understanding of the materials we have and ways to use them and things.
Then Physics and then Biology
Then Physics and then Biology
Original post by Absent Agent
Of course there should be a boundary so that these different fields can be classified, but, as I said, those properties are essentially the knowledge of physics, and if you study them in the field of chemistry, it's simply because you need them.
Not really. Chemistry is literally the study of matter and electrons, protons, neutrons etc are all matter. You could say it also overlaps with physics, but you most certainly can't say it isn't chemistry, because it definitely is.
I've said this before, but even the knowledge of functional groups or elements are just ready-made from the physicist handed over to the chemist. Here is an organic-chemistry data sheet from the web:

I can hardly understand why these data are not ready-made from the physicist.

I can hardly understand why these data are not ready-made from the physicist.
What are you talking about, they have not been handed to chemists by physicists. How organic molecules react and knowledge about their functional groups has been determined by mainly organic chemists and once again, an understanding of physics is not necessary or even particularly useful for an organic chemist.
I never said that it was necessary. Let me quote my first post (although I changed the wording a bit so that it makes sense better) on the thread to which you first replied:
And that's blatantly incorrect, they were and have been possible without any knowledge of quantum mechanics whatsoever. I repeat, QM was only discovered about 100 years ago and organic chemistry has been going on for far longer than that.
These lines summarise what I've been saying, and you can see that I did not imply that knowledge of quantum mechanics was necessary to do chemistry, and I have said that a few times now. What I'm saying is that quantum mechanics has been able to predict the outcomes of reactions and many other chemical properties purely from the knowledge and behavior of subatomic particles.
And I am saying that you can predict reactions and do pretty much all of organic chemistry without any real knowledge on QM. If you're looking at an organic reaction as an organic chemist, knowing wave-particle duality and Heisenberg's uncertainty principle etc is going to be worth sweet nothing when predicting how it will run and in which ways it can be improved.
To build anything you need a knowledge of materials, how they behave and how they interact with each other which can very easily be placed under the field or inorganic and materials chemistry. The machine itself is irrelevant really when talking about what NMR actually does, which depends on the molecules and atoms and is therefore chemistry.
Original post by Plantagenet Crown
And that's blatantly incorrect, they were and have been possible without any knowledge of quantum mechanics whatsoever. I repeat, QM was only discovered about 100 years ago and organic chemistry has been going on for far longer than that.
And I am saying that you can predict reactions and do pretty much all of organic chemistry without any real knowledge on QM. If you're looking at an organic reaction as an organic chemist, knowing wave-particle duality and Heisenberg's uncertainty principle etc is going to be worth sweet nothing when predicting how it will run and in which ways it can be improved.
And I am saying that you can predict reactions and do pretty much all of organic chemistry without any real knowledge on QM. If you're looking at an organic reaction as an organic chemist, knowing wave-particle duality and Heisenberg's uncertainty principle etc is going to be worth sweet nothing when predicting how it will run and in which ways it can be improved.
And that's what exactly what my post says. I stated that QM is able to make predictions just by knowledge of subatomic particles, and not from hypotheses derived by carrying out experiments. How can chemistry predict the outcome of moving a charged particle without prior experimentation?
Original post by Absent Agent
And that's what exactly what my post says. I stated that QM is able to make predictions just by knowledge of subatomic particles, and not from hypotheses derived by carrying out experiments. How can chemistry predict the outcome of moving a charged particle without prior experimentation?
You were talking about theoretically predicting organic reactions which isn't needed for organic chemistry reactions. Again, you can predict by having a basic knowledge of how the elements react, you don't need to know their subatomic, quantum mechanical properties because those properties are different to the ones they have on the big scale. You can know which charges attract and how they will interact with different compounds (i.e. their polarity, electronegativity etc).
Original post by Alex_Rasdra
~wishes the star trek federation political idea was a thing IRL ... space travel, peaceful societies without war and replicators making all my food 
edit... minus the borg maybe

edit... minus the borg maybe
soon ... ~ .-.
Original post by surina16

gotta love maths
 without it biology, chemistry and especially physics would be so much different (and many absent spaces)
without it biology, chemistry and especially physics would be so much different (and many absent spaces)Related discussions
- Does sixth form school matter?
- Bad A level combination
- Advice on choosing degree??!?
- A-level/Career Choice Advice
- University Choice
- computer science- worth it?
- I dunno what subject to drop
- Oxford University for Computer Science
- Which university for Computer science
- royal holloway biomedical science or durham biological sciences???
- computer science at university
- Gcse Options Year 9
- failed History
- Physics and Maths tutor
- how vital is comp sci for a comp sci uni course
- A level 3rd Subject
- How do stand out when applying for a Masters? Comp sci to be specific.
- Triple Science vs Combined Higher
- Physics at oxbridge
- Do you think it should be important to pass GCSE science to go onto A Levels?
Latest
Last reply 1 hour ago
Official University College London Applicant Thread for 2024Last reply 1 hour ago
Can I do economics degree without a level maths?Last reply 1 hour ago
How to choose unis in UCAS application for CS undergraduate course?Last reply 1 hour ago
Rishi Sunak pledges to remove benefits for people not taking jobs after 12 monthsLast reply 2 hours ago
Got my crush's number 2 days ago and no reply yet. What could be the reasons?Posted 2 hours ago
GB News set to axe 40 jobs after channel posts heavy lossesLast reply 2 hours ago
Rwanda bill likely to be stalled at least till April after seven defeats in the LordsPosted 2 hours ago
Sunak rejects offer of youth mobility scheme between EU and UKTrending
Last reply 1 week ago
Is it true that British unis are prejudiced towards degrees from Scottish unis?Last reply 3 weeks ago
Is University of Birmingham prestigious and respected well enough in UK ?Last reply 1 month ago
Do employers care more about the degree subject or the uni you went to?Last reply 1 month ago
Schools failing bright pupils says teen taking 28 a levelsTrending
Last reply 1 week ago
Is it true that British unis are prejudiced towards degrees from Scottish unis?Last reply 3 weeks ago
Is University of Birmingham prestigious and respected well enough in UK ?Last reply 1 month ago
Do employers care more about the degree subject or the uni you went to?Last reply 1 month ago
Schools failing bright pupils says teen taking 28 a levels



