Are atoms the same size?
Heyyy, just wanted some clarification with this chemistry question. I think they arent the same size since atoms belonging to different elements have different sizes whereas atoms of the same element do have the same size. But i think im wrong lol.
Original post by A.N123
Heyyy, just wanted some clarification with this chemistry question. I think they arent the same size since atoms belonging to different elements have different sizes whereas atoms of the same element do have the same size. But i think im wrong lol.
You're right, they aren't all the same size! You can generally assume that the more electrons an atom has, the larger it is going to be.
Original post by Ed5
You're right, they aren't all the same size! You can generally assume that the more electrons an atom has, the larger it is going to be.
Thank You!

Original post by Ed5
You're right, they aren't all the same size! You can generally assume that the more electrons an atom has, the larger it is going to be.
No they are not. In fact, as you move accross a period, the size generally decreases, increasing down groups. So flourine is actually smaller than Lithium etc. Pretty crazy and interesting if you ask me...
Original post by BackLumbarJack
No they are not. In fact, as you move accross a period, the size generally decreases, increasing down groups. So flourine is actually smaller than Lithium etc. Pretty crazy and interesting if you ask me...
BY this I mean They are the same size for the same element and different for different elements.
Original post by Ed5
You're right, they aren't all the same size! You can generally assume that the more electrons an atom has, the larger it is going to be.
Not strictly true, sorry.
Atoms of an element are the same size as each other. They are different sizes from atoms of other elements.
However, atoms increase in size with increasing number of electron shells.
When comparing elements with the same number of electron shells (i.e. those in the same period, the same horizontal row of the periodic table) the atomic size decreases with increasing number of electrons. You don't need to understand why until A level.
Original post by BackLumbarJack
...
Original post by TutorsChemistry
...
Thanks for the tips, but OP is studying at GCSE level so I didn't want to throw in any unnecessary confusion! When I said generally, I was referring to the fact that the overall trend is an increase, ignoring the small deviations from this when filling different types of orbital etc.
Original post by Ed5
Thanks for the tips, but OP is studying at GCSE level so I didn't want to throw in any unnecessary confusion! When I said generally, I was referring to the fact that the overall trend is an increase, ignoring the small deviations from this when filling different types of orbital etc.
No, you are still wrong.
There are two trends:
If you add more e-, without starting to fill a new shell, the radius decreases.
If you add more e-, which leads to a new shell being filled, the radius increases.
(As TutorsChemistry said IOW)
(edited 6 years ago)
Original post by Pigster
No, you are still wrong.
There are two trends:
If you add more e-, without starting to fill a new shell, the radius decreases.
If you add more e-, which leads to a new shell being filled, the radius increases.
(As TutorsChemistry said IOW)
There are two trends:
If you add more e-, without starting to fill a new shell, the radius decreases.
If you add more e-, which leads to a new shell being filled, the radius increases.
(As TutorsChemistry said IOW)
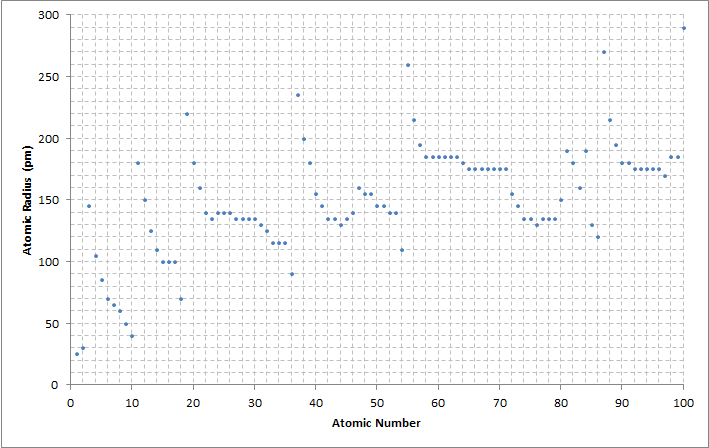
It's generally an increasing trend - this is what I meant in my original post, so I'm sorry if that wasn't communicated clearly. This wasn't even part of OP's question, I just threw it in there for some extra knowledge, idk what all the fuss is about.
(edited 6 years ago)
Thanks everyone! 
 Sorry if my question caused a little argument.....
Sorry if my question caused a little argument.....

 Sorry if my question caused a little argument.....
Sorry if my question caused a little argument.....Original post by Ed5
It's generally an increasing trend - this is what I meant in my original post, so I'm sorry if that wasn't communicated clearly. This wasn't even part of OP's question, I just threw it in there for some extra knowledge, idk what all the fuss is about.

It's generally an increasing trend - this is what I meant in my original post, so I'm sorry if that wasn't communicated clearly. This wasn't even part of OP's question, I just threw it in there for some extra knowledge, idk what all the fuss is about.
I presume the fuss is about accuracy of the words we say to things they are supposed to apply to.
For certain numbers of electrons you add, you increase the atomic radius, yes.
For certain other numbers of electrons you add, you decrease the atomic radius.
For a reader who doesn't understand the context of these opposite effects, a coverall statement is probably more harmful than helpful, because at best they are wrong half the time, and at worst they feel like they understand a phenomenon that they certainly don't, which could bias their learning in the future.
I'd advise that more information is always better than less, and that specific statements that can't be taken out of context are also good

Quick Reply
Related discussions
- Electronegativity - A level AQA
- homework help
- What is the difference between simple molecular and simple covalent substances?
- Chemistry - solubility and enthalpy of hydration
- Chemistry electronegativity
- Hydrogen bonds A level
- A level chemistry why does reaction of decomposing BaCO3 take longer than MgCO3
- Mass vs relative atomic mass on periodic table
- Bond breaking and intermolecular force A level
- Chemistry question organic
- Chemistry help urgent Alevel
- Chemistry
- Moles in A-Level Chemistry
- Boiling point of halogenoalkanes
- Chemistry help
- Edexcel GCSE Chemistry Paper 1 Higher Tier 1CH0 1H - 27 May 2022 [Exam Chat]
- Alevel physics
- A level chemistry optical isomerism MC questions
- chemistry combustion help
- A level combustion
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



