Exothermic reactions and energy
Why does increasing the temperature of an exothermic reaction shift the position of equilibrium to the left?
Eg, with all species in gaseous form:
CO(g)+2H₂(g)⇌CH₃OH(g) ∆H=-94kJmol⁻¹
Please also don't give me similes - I'm trying to use an explanation that involves the increase in energy the system gets to explain this so that it makes full sense to me 🙂.
Eg, with all species in gaseous form:
CO(g)+2H₂(g)⇌CH₃OH(g) ∆H=-94kJmol⁻¹
Please also don't give me similes - I'm trying to use an explanation that involves the increase in energy the system gets to explain this so that it makes full sense to me 🙂.
temp increases
position of equilibrium needs to shift in a way that will reduce this change to maintain equilibrium
it shifts in the endothermic direction
left side is endothermic so position shifts to the left
position of equilibrium needs to shift in a way that will reduce this change to maintain equilibrium
it shifts in the endothermic direction
left side is endothermic so position shifts to the left
Thank you, but I'm asking why it shifts in the wae of the endothermic reaction 🙂
Original post by Pigster
The forward reaction (being exothermic) involves energy being released (more energy released forming bonds than taken in breaking others). IF you raise the temperature and IF the forward reaction was favoured, the reaction would get even hotter. Not sensible.
That's actually a good point! 😄 ; although, sorry but it still doesn't give a good answer - the reaction doesn't have a brain of its own and can't decide which path to go down 😄
Original post by Pigster
The forward reaction (being exothermic) involves energy being released (more energy released forming bonds than taken in breaking others). IF you raise the temperature and IF the forward reaction was favoured, the reaction would get even hotter. Not sensible.
Plus, if it did, the military would already be hot on investigation! 😂
Original post by Freedom physics
Why does increasing the temperature of an exothermic reaction shift the position of equilibrium to the left?
Eg, with all species in gaseous form:
CO(g) 2H₂(g)⇌CH₃OH(g) ∆H=-94kJmol⁻¹
Please also don't give me similes - I'm trying to use an explanation that involves the increase in energy the system gets to explain this so that it makes full sense to me 🙂.
Eg, with all species in gaseous form:
CO(g) 2H₂(g)⇌CH₃OH(g) ∆H=-94kJmol⁻¹
Please also don't give me similes - I'm trying to use an explanation that involves the increase in energy the system gets to explain this so that it makes full sense to me 🙂.
Any eqm reaction wants to oppose any change made to it. As the forward reaction is exothermic (releases energy), the reaction naturally wants to take in energy to oppose the change (and therefore favouring the endothermic/backward reaction). As a result, further increasing the temperature means that the reaction will want to take in even more energy and therefore shifts eqm even further to the side of the endothermic reaction which is left
(edited 5 years ago)
Original post by Freedom physics
Thank you, but I'm asking why it shifts in the way of the endothermic reaction 🙂
It is a function of the Maxwell Boltzmann distribution and the activation energy of both forward and back processes in the equilibrium.
For an endothermic reaction the activation energy of the endothermic process is necessarily lower than that of the exothermic direction.
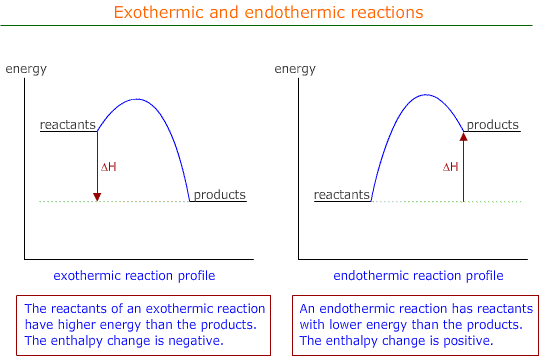
Hence, the activation energy of the endothermic process on the Maxwell Boltzmann is to the higher energy side.
Equilibrium is the point at which the rate of the forward reaction equals the rate of the back reaction and both of these are a function of the concentration of the reacting species with sufficient activation energy.
An increase in temperature proportionally affects the population of activated particles by a larger percentage at the higher end of the Maxwell Boltmann curve. Hence, the number of particles with sufficient activation energy is affected more for the endothermic change then the exothermic change and the equilibrium moves towards the direction of endothermic change.
Original post by TODTEMPLE01
Any eqm reaction wants to oppose any change made to it. As the forward reaction is exothermic (releases energy), the reaction naturally wants to take in energy to oppose the change (and therefore favouring the endothermic/backward reaction). As a result, further increasing the temperature means that the reaction will want to take in even more energy and therefore shifts eqm even further to the side of the endothermic reaction which is left
Thank you; that explanation is partially satisfying and partially explains it and is enough to have me accept it 🙂 - the part which i don't like is just the le chateliers principle part of it which states the reaction wants to oppose any changes and thus keep the system how it is 🙂
Original post by Freedom physics
Thank you; that explanation is partially satisfying and partially explains it and is enough to have me accept it 🙂 - the part which i don't like is just the le chateliers principle part of it which states the reaction wants to oppose any changes and thus keep the system how it is 🙂
As you said earlier, reactions don't 'want' anything. Expressing the lCP in anthropomorphic terms just makes it easier to explain. As charco said, the actual explanation is all to do with the Maxwell Boltzmann distribution. For the endothermic process (with a high Ea), perhaps only 10% of collisions have sufficient energy and for the exothermic process (with lower Ea) maybe 30% of collisions have enough energy. Now, when you raise T, both curves shift right (and down), so for both curves there are now more particles with E>Ea. It might be that now there is 11% and 32% with E>Ea. BUT the 11% represents a 10% increase (from 10 to 11%) and the other represents a 6.7% increase (why did I pick horrible numbers?). The endothermic reaction is proportionally sped up more than the exothermic one.
Quick Reply
Related discussions
- bio help a level
- le chapeliers principle, exo and endo?????
- URGENT chem question help bond enthalpies
- chemistry help
- Remembering Exothermic and Endothermic
- thermochemistry
- Chemistry Physical question enthalpy of solution
- Chemistry question
- GCSE Chemistry Triple Science
- Chemistry Olympiad question
- equilibriums in reversible reactions
- A Level Chemistry HELP
- Hard Enthalpy Change Question - Help Needed
- Aqa a level chem enthalpy help!!
- Hess law and combustion
- enthalpy change of solution
- 2Al(s) Fe2O3(s) → Al2O3(s) 2Fe(s), struggling with this question
- AQA GCSE Combined Science Paper 2 Higher Tier (8464/C/2H) -13th June 2023 [Exam Chat]
- enthalpy change
- AQA GCSE Chemistry Paper 2 (Higher Tier) 8462/2H - 20 Jun 2022 [Exam Chat]
Latest
Last reply 1 minute ago
Government Social Research - Research Officer Scheme 2024Last reply 1 minute ago
Civil Service Fast Stream 2024 - Applicants threadLast reply 3 minutes ago
TSR Study Together - STEM vs Humanities - Tenth SessionLast reply 3 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 6 minutes ago
Imperial vs UCL vs KCL for PhysicsLast reply 6 minutes ago
Bank of America Degree Apprenticeship 2024Last reply 7 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 10 minutes ago
LSE Economic history departmentLast reply 12 minutes ago
Medical doctor degree apprenticeship 2024Last reply 13 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 14 minutes ago
Official University of Warwick Offer Holders Thread for 2024 entryTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products



