Edexcel A2 Chemistry 6ch04/05 JUNE 2015
Scroll to see replies
Original post by Nautic4l
http://qualifications.pearson.com/content/dam/pdf/A%20Level/Chemistry/2013/Exam%20materials/6CH04_01_que_20110615.pdf
Can anyone help with 5 and 12 please? With explanation?
Can anyone help with 5 and 12 please? With explanation?
This is how I did q5. Work out kc of both then you can see that if you square kc of the 2nd equation you get kc of the first. Hope this helps.☺️
Original post by Nautic4l
http://qualifications.pearson.com/content/dam/pdf/A%20Level/Chemistry/2013/Exam%20materials/6CH04_01_que_20110615.pdf
Can anyone help with 5 and 12 please? With explanation?
Can anyone help with 5 and 12 please? With explanation?
for question 12 the answer i think is B.
it cant be C or D as their is no chiral carbon present
it cannot be A as the priority groups are on opposite sides so that would be an E isomer
Original post by MeeraP07
do you happen to have a question to make the explanation a bit easier?
Hi, so a example would be: http://qualifications.pearson.com/content/dam/pdf/A%20Level/Chemistry/2013/Exam%20materials/6CH04_01_que_20130114.pdf
question 27)ii)
COuld you please show me your working behind it?
Original post by Leeki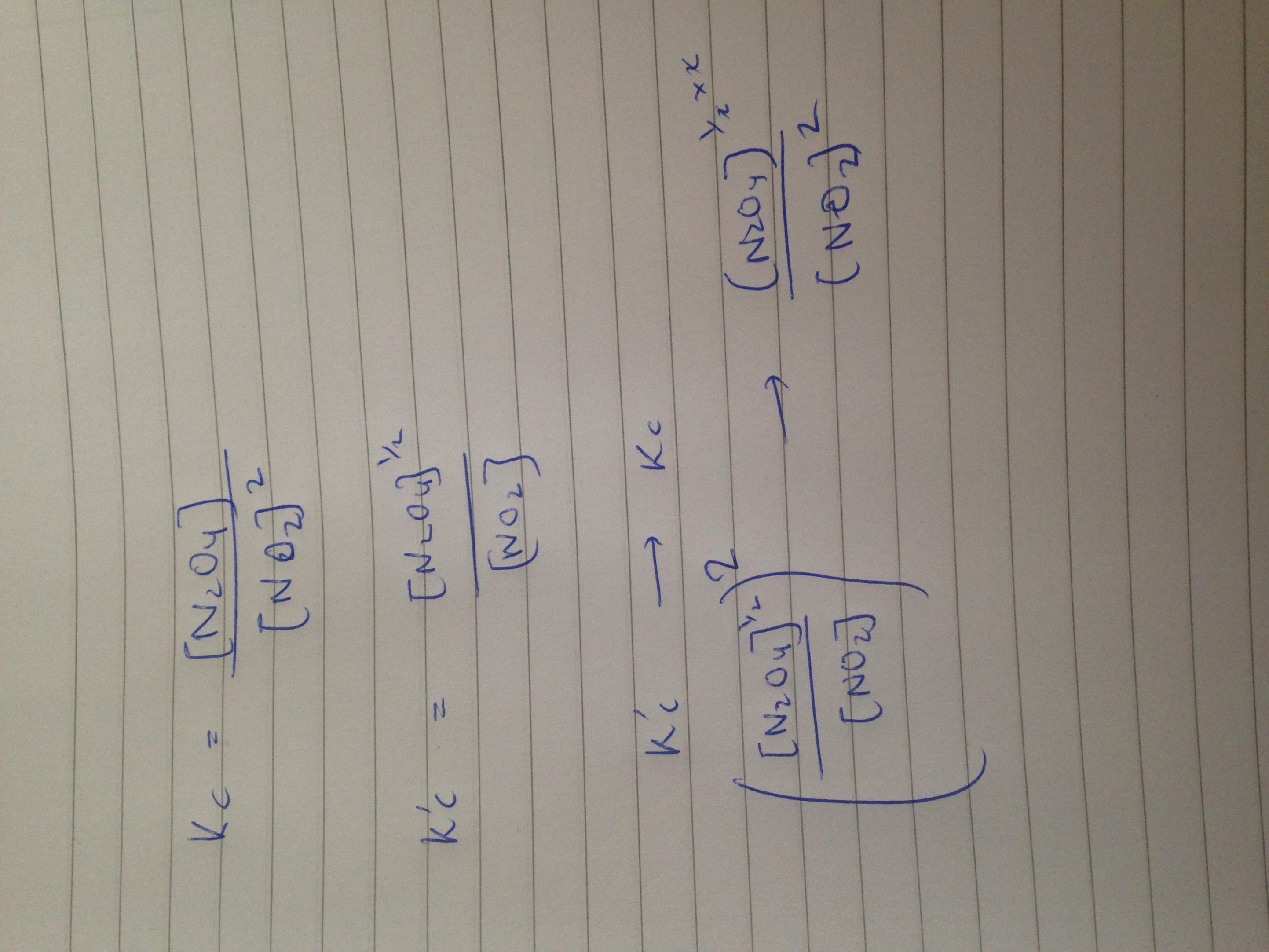
This is how I did q5. Work out kc of both then you can see that if you square kc of the 2nd equation you get kc of the first. Hope this helps.☺️
This is how I did q5. Work out kc of both then you can see that if you square kc of the 2nd equation you get kc of the first. Hope this helps.☺️
Brilliant. Thank you very much

Original post by simsid
for question 12 the answer i think is B.
it cant be C or D as their is no chiral carbon present
it cannot be A as the priority groups are on opposite sides so that would be an E isomer
it cant be C or D as their is no chiral carbon present
it cannot be A as the priority groups are on opposite sides so that would be an E isomer
Can you explain more please? Not sure how to go about those type of questions at all.
So how do you know what the priority groups are? And how did you eliminate some options? Chiral carbon has 4 different groups yeah? How do you know that from the skeletal formula?
guys im really scared about soap making/ transesterificastion... also when they throw in random stuff like the june 2014 papr!!!
e=volume of solution */_\T* c
how was i suppoed to know you fonr convert to dmcubed!!!!!!!!!
so yeah guys do you have any resources or good techniques to do them (transesfterifcation/soap making)???
thank alot and good luck
e=volume of solution */_\T* c
how was i suppoed to know you fonr convert to dmcubed!!!!!!!!!

so yeah guys do you have any resources or good techniques to do them (transesfterifcation/soap making)???
thank alot and good luck
Original post by madmenace
guys im really scared about soap making/ transesterificastion... also when they throw in random stuff like the june 2014 papr!!!
e=volume of solution */_\T* c
how was i suppoed to know you fonr convert to dmcubed!!!!!!!!!
so yeah guys do you have any resources or good techniques to do them (transesfterifcation/soap making)???
thank alot and good luck
e=volume of solution */_\T* c
how was i suppoed to know you fonr convert to dmcubed!!!!!!!!!

so yeah guys do you have any resources or good techniques to do them (transesfterifcation/soap making)???
thank alot and good luck
page 14 https://chemrevise.files.wordpress.com/2015/06/5-further-organic.pdf
Has anyone done the June 2013 R paper that would mind answering a few questions? (:
Original post by Nautic4l
Brilliant. Thank you very much 
Can you explain more please? Not sure how to go about those type of questions at all.
So how do you know what the priority groups are? And how did you eliminate some options? Chiral carbon has 4 different groups yeah? How do you know that from the skeletal formula?

Can you explain more please? Not sure how to go about those type of questions at all.
So how do you know what the priority groups are? And how did you eliminate some options? Chiral carbon has 4 different groups yeah? How do you know that from the skeletal formula?
Original post by Nautic4l
Brilliant. Thank you very much 
Can you explain more please? Not sure how to go about those type of questions at all.
So how do you know what the priority groups are? And how did you eliminate some options? Chiral carbon has 4 different groups yeah? How do you know that from the skeletal formula?

Can you explain more please? Not sure how to go about those type of questions at all.
So how do you know what the priority groups are? And how did you eliminate some options? Chiral carbon has 4 different groups yeah? How do you know that from the skeletal formula?
if you cant tell from the skeletal formula your just going to have to draw out the displayed formula.
priority groups are the groups either side of the double bond with the highest molar mass basically (this stuff was in AS so try and find some resources if your not sure.)
Original post by goonieskellie
Has anyone done the June 2013 R paper that would mind answering a few questions? (:
I did that paper. I don't mind.
Original post by Leeki
Can anyone please explain how a buffer solution works like with an example? 😥 I feel like that's gonna be one of the questions...everything I don't know always comes up.😥 thanks ☺️
this is really useful.
Original post by simsid
http://www.chemguide.co.uk/physical/acidbaseeqia/buffers.html
this is really useful.
this is really useful.
Thanks ! ☺️😊
Original post by Leeki
I did that paper. I don't mind.
Ah ok awesome, they are quite simple questions i think but i just can't understand them aha!
11.b.i. how do you get to 0.05?
12.b. how do you know what the equivalence point is?
13.b.iv. i wrote 2.4 and 4.8 and i dont really understand why both the answers are 2.4. do you measure the distance between the half lives?
thankyou! i know most of them are probably really obvious lol :l
Original post by cathalmcc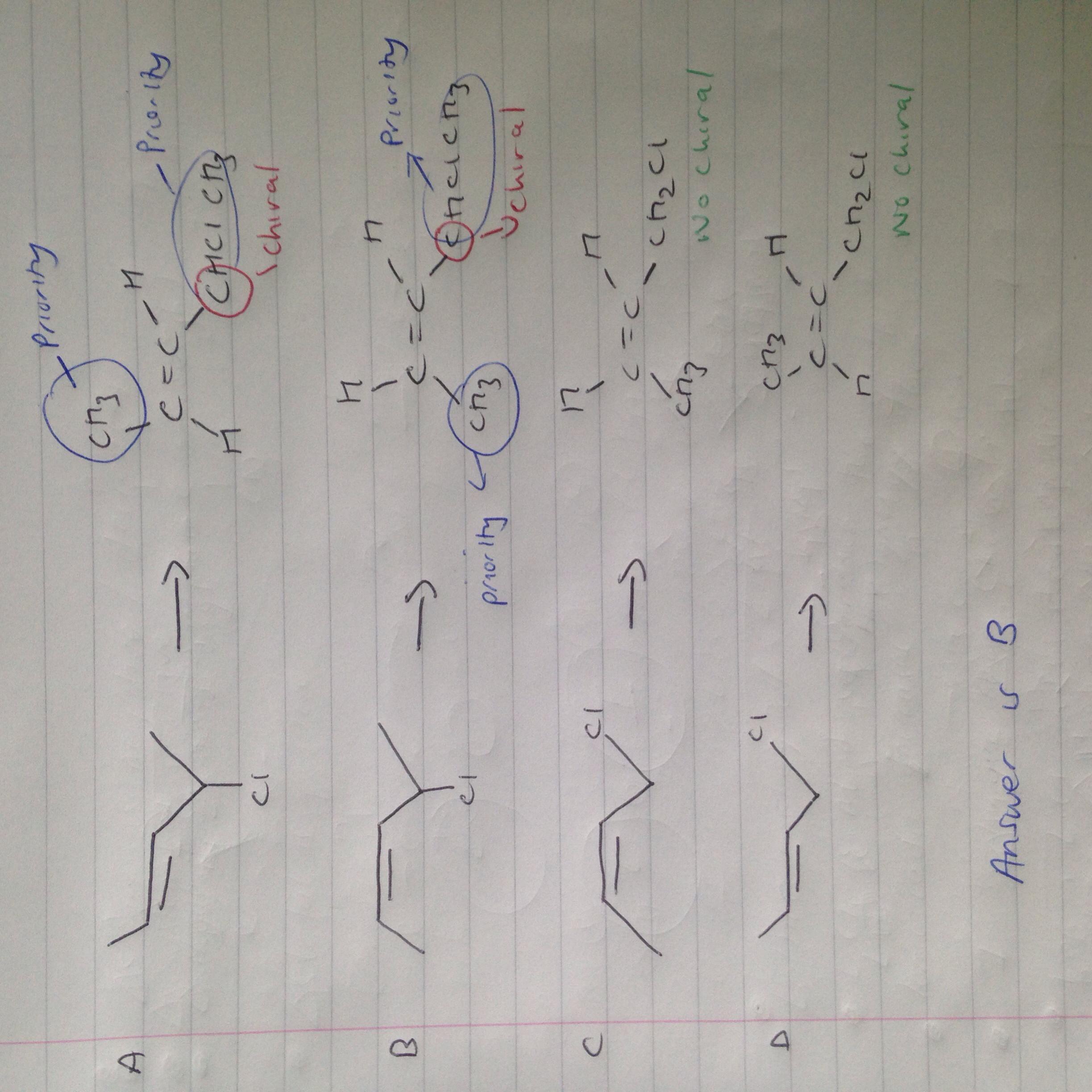 Hope this helps 👍👍
Hope this helps 👍👍
Perfect, thank you

http://qualifications.pearson.com/content/dam/pdf/A%20Level/Chemistry/2013/Exam%20materials/6CH04_01_que_20110126.pdf
Questions wrt Bronsted-Lowry acid-base like Q13, what do you guys look for immediately? I always get them wrong
Questions wrt Bronsted-Lowry acid-base like Q13, what do you guys look for immediately? I always get them wrong
At 100°C, pure water has a pH of 6, whereas at 25°C it has a pH of 7. This is because
A the dissociation of water is endothermic, so the concentration of hydrogen ions is lower at 100 °C than it is at 25 °C.
B the dissociation of water is exothermic, so the concentration of hydrogen ions is lower at 100°C than it is at 25°C.
C the dissociation of water is endothermic, so the concentration of hydrogen ions is higher at 100 °C than it is at 25 °C.
D at 100 °C, water has a higher concentration of hydrogen ions than of hydroxide ions.
the answer is C but how do you know that it would be C?
A the dissociation of water is endothermic, so the concentration of hydrogen ions is lower at 100 °C than it is at 25 °C.
B the dissociation of water is exothermic, so the concentration of hydrogen ions is lower at 100°C than it is at 25°C.
C the dissociation of water is endothermic, so the concentration of hydrogen ions is higher at 100 °C than it is at 25 °C.
D at 100 °C, water has a higher concentration of hydrogen ions than of hydroxide ions.
the answer is C but how do you know that it would be C?
Original post by Nautic4l
http://qualifications.pearson.com/content/dam/pdf/A%20Level/Chemistry/2013/Exam%20materials/6CH04_01_que_20110126.pdf
Questions wrt Bronsted-Lowry acid-base like Q13, what do you guys look for immediately? I always get them wrong
Questions wrt Bronsted-Lowry acid-base like Q13, what do you guys look for immediately? I always get them wrong
I always look at what molecule has lost a hydrogen and which one has gained a hydrogen. the answer is A because the OH gains a CH3 group so this is not a acid base reaction
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Changes to the A-Level course?
- GCSE Exam Discussions 2023
- School is killing me - Y11 "GYG" 2022
- Do I have to retake the year or can I just resit the exam?
- A Level Exam Discussions 2023
- AQA as level economics 2015 specimen paper 2
- Edexcel GCSE Combined Sci Paper 2 Foundation (1SC0 2CF) - 13th June 2023 [Exam Chat]
- IAL repeats cash in.
- Required practicals chemistry A level
- Self-teaching Chemistry A-level (as a private candidate)?
- 1000+ A2-Level Biology Exam Questions
- Switching from A level to IAL
- Audio files for GCSE French Listening??
- Is 4 months enough time to get a 7 in GCSE Maths and Chemistry
- GCSE Diary
- Edexcel A Level Biology B Paper 3: 9BI0 03 - 24 Jun 2022 [Exam Chat]
- Ial edexcel notes/resource
Latest
Trending
Last reply 7 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 7 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]


