Shapes of complex ions
Cis-platin has the 'square planar' shape, whereas copper with four chlorides around it has a tetrahedral shape, according to a mark scheme I have. I don't understand why there is a difference or why the square planar shape occurs as I haven't encountered it before. Someone shed some light on complex ions with four monodentate ligands?
Much love
Much love

Thanks 

As has been said, you don't need to know for A level. The reason square planar complexes occur is due to the way the metal d orbitals get lowered/raised in energy as a result of interactions with ligands. Different geometeries cause different arrangements of orbitals energy wise, and then the number of electrons you have to fill them with determines which configuration is adopted.
eg:
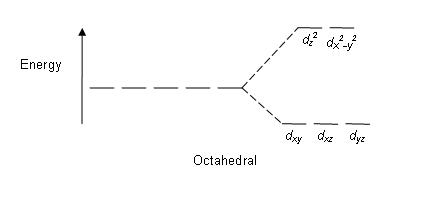


eg:



Quick Reply
Related discussions
- Will the bond angle in H2O(104.5) increase if water is bind to other molecules?
- Complex ions showing optical isomerism question
- Help chemistry alevel
- Chemistry - transition metals
- Transition metal complexes and water of crystallisation/water ligands.
- 6CH05/01 june 2013
- Alevel chemistry
- Ligand substitution
- colours of salts?
- Have i explained how the Bohr effect can impact the structure of haemoglobin correct?
- Shapes of molecules
- Transition Metal Ions
- Chemistry transition metals
- AQA A Level Chemistry Transition metals
- titrating calcium ions
- Aqa a level chemistry paper 1 2017 question 7.5
- Why r bicarbonate ions removed from red blood cells
- chemistry help please
- Chemistry Questions
- Mercury I Chloride, Ionic or Covalent?
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



