Oxidation of alkene...
Please read my whole problem to know why my answer is different from mark scheme.
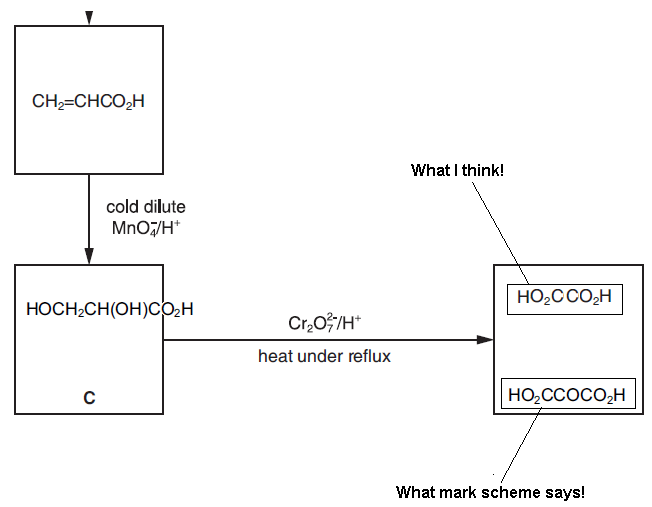
I know that primary alcohols are oxidised to acids and secondary alcohols are oxidised to ketones on refluxing, but I think as that diol is coming from an alkene, so the same would happen with it on refluxing with K2Cr2O7, as it happens with an alkene when it is completely oxidised by Hot, Concentrated, Acidified KMnO4.
I was taught that oxidation of alkene with Hot, Concentrated, Acidified KMnO4 happens like this:
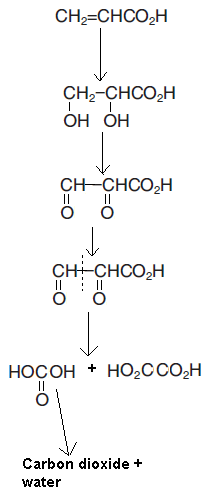
Diol is also formed in this process and here 'what I think' is formed, so am I not right?
Or IS markscheme right because the diol is then oxidsied by K2Cr2O7?

I know that primary alcohols are oxidised to acids and secondary alcohols are oxidised to ketones on refluxing, but I think as that diol is coming from an alkene, so the same would happen with it on refluxing with K2Cr2O7, as it happens with an alkene when it is completely oxidised by Hot, Concentrated, Acidified KMnO4.
I was taught that oxidation of alkene with Hot, Concentrated, Acidified KMnO4 happens like this:

Diol is also formed in this process and here 'what I think' is formed, so am I not right?
Or IS markscheme right because the diol is then oxidsied by K2Cr2O7?
(edited 13 years ago)
In Box C you have a molecule with a primary alcohol group and a secondary alcohol group.
The primary alcohol group get oxidised first to an aldehyde the a carboxylic acid.
The secondary alcohol group becomes a ketone.
Lastly, there are no double bonds in the molecule for clevage to occur/diols to form. You cant expect KMnO4 to work in the same manner as Acidifed dichromate.
The primary alcohol group get oxidised first to an aldehyde the a carboxylic acid.
The secondary alcohol group becomes a ketone.
Lastly, there are no double bonds in the molecule for clevage to occur/diols to form. You cant expect KMnO4 to work in the same manner as Acidifed dichromate.
Original post by Ari Ben Canaan
In Box C you have a molecule with a primary alcohol group and a secondary alcohol group.
The primary alcohol group get oxidised first to an aldehyde the a carboxylic acid.
The secondary alcohol group becomes a ketone.
Lastly, there are no double bonds in the molecule for clevage to occur/diols to form. You cant expect KMnO4 to work in the same manner as Acidified dichromate.
The primary alcohol group get oxidised first to an aldehyde the a carboxylic acid.
The secondary alcohol group becomes a ketone.
Lastly, there are no double bonds in the molecule for clevage to occur/diols to form. You cant expect KMnO4 to work in the same manner as Acidified dichromate.
Nope. I'm pretty sure that when a double bonded carbon atom has one Hydrogen attached to it, it always forms acid, and if it has no hydrogen attached to it, it always forms a ketone. And if it has 2 hydrogens attached to it, it forms carbon dioxide and water. And this all happens with Hot, conc, acidified KMnO4. :/
This doesn't happens when a particular diol is oxidised by acidified Potassium dichromate.
(edited 13 years ago)
Your second scheme doesn't really reflect how permanganate oxidises alkenes 
As said above the mechanims of oxidation are quite distinct between the two oxidants, permanganate is prone to causing oxidative cleavage whilst dichromate isn't. If you're feeling brave take a look at how permanganate acts

As said above the mechanims of oxidation are quite distinct between the two oxidants, permanganate is prone to causing oxidative cleavage whilst dichromate isn't. If you're feeling brave take a look at how permanganate acts

(edited 13 years ago)
Reckon the mark scheme may be correct.
Your answer is wrong because you are missing out one of the carbons. Oxidation & reduction don't affect carbons; mainly oxygen and hydrogen atoms.
The alkene is first oxidised to a diol; but also has another -OH on the central carbon. When further oxidised; especially with dichromate under reflux, complete oxidation occurs. So the 2 -OH groups at both extremes of the molecule get oxidised to carboxylic acid groups. The -OH on the central carbon is bonded in a "secondary alcohol position" and so will also be oxidised, this time to a ketone group -CO.
Remember with reference to organic chem:
Oxidation = addition of O/removal of H
Reduction =addition of H/ removal of O
Your answer is wrong because you are missing out one of the carbons. Oxidation & reduction don't affect carbons; mainly oxygen and hydrogen atoms.
The alkene is first oxidised to a diol; but also has another -OH on the central carbon. When further oxidised; especially with dichromate under reflux, complete oxidation occurs. So the 2 -OH groups at both extremes of the molecule get oxidised to carboxylic acid groups. The -OH on the central carbon is bonded in a "secondary alcohol position" and so will also be oxidised, this time to a ketone group -CO.
Remember with reference to organic chem:
Oxidation = addition of O/removal of H
Reduction =addition of H/ removal of O
Original post by EierVonSatan
Your second scheme doesn't really reflect how permanganate oxidises alkenes 
As said above the mechanims of oxidation are quite distinct between the two oxidants, permanganate is prone to causing oxidative cleavage whilst dichromate isn't. If you're feeling brave take a look at how permanganate acts

As said above the mechanims of oxidation are quite distinct between the two oxidants, permanganate is prone to causing oxidative cleavage whilst dichromate isn't. If you're feeling brave take a look at how permanganate acts

Oh, wow! Thanks a lot for that.
 I'm brave in the matter of studies.
I'm brave in the matter of studies. 
Quick Reply
Related discussions
- chemistry help
- Alevel Chemistry HELP
- Does anyone why the answer to this a level chemistry question is A
- Reaction Mechanism of Allyl Bromide and (aqueous) NaOH
- The suffix en for alkenes
- year 12 study journal!
- OCR A-Level Chemistry Synthetic Routes
- Organic chemistry reaction
- Organic chemistry
- AS Organic Chem Question
- Chemistry alevel mechanisms
- question about a half equation
- Organic chemistry question
- Calculate the oxidation state of the vanadium ion?! URGENT
- how do i suggest the formula for sodium chlorate (vii) ??
- chemistry combustion help
- A Level Chemistry Exam Question-need help!
- A level chemistry Redox help please.
- Oxidation number format vs ion charge format
- Acidic alkaline basic metals and non metals
Latest
Last reply 1 hour ago
Official University College London Applicant Thread for 2024Last reply 1 hour ago
Can I do economics degree without a level maths?Last reply 1 hour ago
How to choose unis in UCAS application for CS undergraduate course?Last reply 1 hour ago
Rishi Sunak pledges to remove benefits for people not taking jobs after 12 monthsLast reply 2 hours ago
Got my crush's number 2 days ago and no reply yet. What could be the reasons?Posted 2 hours ago
GB News set to axe 40 jobs after channel posts heavy lossesPosted 2 hours ago
Sunak rejects offer of youth mobility scheme between EU and UK



