AQA CHEM2 ~ May 23rd 2012 ~ AS Chemistry
Scroll to see replies
Original post by Mocking_bird
I have more than that on just the first 2 pages of my booklets 
Will type them up later maybe so somebody can hopefully tell me i'm learning way more than i need to,

Will type them up later maybe so somebody can hopefully tell me i'm learning way more than i need to,

those are the key ones you need to learn

Original post by Mocking_bird
So this is too much then?
Extraction of Iron
3) Carbon monoxide acts as a reducing agent and reduces most of Iron(III) oxide.
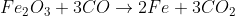
4) Coke also acts as a reducing agent in the hotter area of the furnace at the bottom.
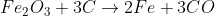
Extraction of Iron
3) Carbon monoxide acts as a reducing agent and reduces most of Iron(III) oxide.
4) Coke also acts as a reducing agent in the hotter area of the furnace at the bottom.
For extraction of iron, only those two are needed

Also, you can cut down the no. of equations you need to remember by just remembering the general equation:
Metal Oxide + carbon/carbon monoxide --> Metal + carbon dioxide
and just remember the exceptions
(edited 12 years ago)
Original post by Mocking_bird
So this is too much then?
Extraction of Iron
1) Coke reacts with hot air - very exothermic, responsible for high temps in furnace
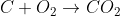
2) Carbon dioxide at high temps reacts with unreacted coke
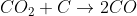
3) Carbon monoxide acts as a reducing agent and reduces most of Iron(III) oxide.
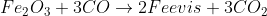
4) Coke also acts as a reducing agent in the hotter area of the furnace at the bottom.
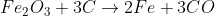
5) Limestone is used to remove impurities. Limestone first decomposes.
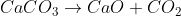
6) Calcium oxide produced is a basic oxide and reacts with acidic oxides.
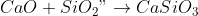
Extraction of Copper:
Original method:
1) Reduce ore to copper oxide by heating
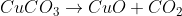
2) Oxide heated with coke
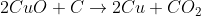
3) Overall equation:
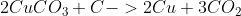
Recent way:
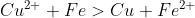 .
.
Extraction of Maganese:
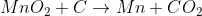
Extraction of Tungsten:
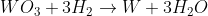
So thats not including extraction of aluminium (4 equations), titanium(2 equations), carbide formation (1) , and 3 pollution problem equations. [Didnt want to type them out as if i've not learnt that far yet].
If ive just learnt all them for no reason my teacher is evil for giving us this booklet to learn on our own
Extraction of Iron
1) Coke reacts with hot air - very exothermic, responsible for high temps in furnace
2) Carbon dioxide at high temps reacts with unreacted coke
3) Carbon monoxide acts as a reducing agent and reduces most of Iron(III) oxide.
4) Coke also acts as a reducing agent in the hotter area of the furnace at the bottom.
5) Limestone is used to remove impurities. Limestone first decomposes.
6) Calcium oxide produced is a basic oxide and reacts with acidic oxides.
Extraction of Copper:
Original method:
1) Reduce ore to copper oxide by heating
2) Oxide heated with coke
3) Overall equation:
Recent way:
Extraction of Maganese:
Extraction of Tungsten:
So thats not including extraction of aluminium (4 equations), titanium(2 equations), carbide formation (1) , and 3 pollution problem equations. [Didnt want to type them out as if i've not learnt that far yet].
If ive just learnt all them for no reason my teacher is evil for giving us this booklet to learn on our own

i dont think they ask you about the extraction of copper though i would check the specification, same with maganese.
Original post by cuckoo99
i dont think they ask you about the extraction of copper though i would check the specification, same with maganese.
"understand that carbon and carbon monoxide are cheap
and effective reducing agents that are used in the extraction
of iron, manganese and copper (reduction equations and
conditions only)"
So looks like I can get rid of a fair amount of the Iron, like pointed out above. & Would it just be the "recent" equation I need for Copper, then Manganese just how it is up there.
Oh I dont know
 I've done too much work today
I've done too much work today 
Original post by cuckoo99
i dont think they ask you about the extraction of copper though i would check the specification, same with maganese.
They are needed
Original post by MedicalMayhem
Ah okay, so in an exam that'd be okay? What's the other thing talking about then, with the sulphate ions? Is that equation correct as well?
I would advise you do it as you first thought. Although, both are correct. It's to do with supply of protons and other acids involved, but you don't need to concern yourself with it.
(edited 12 years ago)
I've finished this unit but due to my lack of attention during lessons, I don't really understand anything  Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
 Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
Going to reteach myself the whole Unit commencing now! How long do you think it'll take?Original post by Member737,514
I've finished this unit but due to my lack of attention during lessons, I don't really understand anything  Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
 Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
Going to reteach myself the whole Unit commencing now! How long do you think it'll take?I don't mean to be rude, but how can we possibly answer that kind of a question? It's dependent on so much that we don't know.
I see this so often on TSR.
Original post by Tullia
I don't mean to be rude, but how can we possibly answer that kind of a question? It's dependent on so much that we don't know.
I see this so often on TSR.
I see this so often on TSR.
Fair enough! I guess it was a poorly worded, vague question, I just mean if anyone could speak from their own experience perhaps it would give me a rough-ish estimate.
Original post by Member737,514
Fair enough! I guess it was a poorly worded, vague question, I just mean if anyone could speak from their own experience perhaps it would give me a rough-ish estimate.
Buy the CGP book
~2-3 weeks probs
Original post by Member737,514
I've finished this unit but due to my lack of attention during lessons, I don't really understand anything  Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
 Going to reteach myself the whole Unit commencing now! How long do you think it'll take?
Going to reteach myself the whole Unit commencing now! How long do you think it'll take?4 hours over 2-3 days should suffice for off-by-heartedness
 .
.-would you really neg me for this? Its serious advice, 4 hours each day for 2-3 days should suffice on learning the Extraction Unit. It is just memory.
(edited 12 years ago)
Original post by cuckoo99
quick question for people following this thread: what did every1 get in their unit 1 exam and are you retaking that aswell as doing the unit 2 exam?
I got a grade I'm happy with so I'm not resitting

Original post by ElMoro
I got a grade I'm happy with so I'm not resitting 

what grade was that
 and good to hear
and good to hearOriginal post by cuckoo99
quick question for people following this thread: what did every1 get in their unit 1 exam and are you retaking that aswell as doing the unit 2 exam?
100 ums

if you put the time in then you'll get your marks. You get out what you put in and such.
A love for the subject helps greatly too.
Original post by Silverland
100 ums 
if you put the time in then you'll get your marks. You get out what you put in and such.
A love for the subject helps greatly too.

if you put the time in then you'll get your marks. You get out what you put in and such.
A love for the subject helps greatly too.
nice 1!! i was going through a rough time during the exam period and didnt revise enough :/ hoping to smash the exam this time round. i love chemistry
 hoping to do a chemistry related degree at uni or just chemistry ;p;p
hoping to do a chemistry related degree at uni or just chemistry ;p;pQuick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- Edexcel chemistry unit 2 mixed questions
- AQA A Level Law Paper 1 (7162/1) - 23rd May 2024 [Exam Chat]
- AQA A Level History Paper 1 (7042/1A-1L) - 23rd May 2024 [Exam Chat]
- SQA Exam Discussions 2024
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- School is killing me - Y11 "GYG" 2022
- AQA A-level English Language Paper 1 (7702/1) - 23rd May 2024 [Exam Chat]
- SQA Nat 5 Chemistry - 23rd May 2024 [Exam Chat]
- AQA A-level English Lang
- A-level Chemistry Study Group 2022-2023
- SQA Higher Chemistry - Paper 2 - 23rd May 2024 [Exam Chat]
- SQA Advanced Higher Chemistry - 23rd May 2024 [Exam Chat]
- SQA Higher Chemistry - Paper 1 Multiple Choice - 23rd May 2024 [Exam Chat]
- A-level Business Study Group 2022-2023
- English Lit Paper 2 leak??!?!!
- AS chemistry past paper help
Latest
Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



