OCR AS Salters Chemistry F332 - Wednesday 23rd May 2012 1:30pm
Scroll to see replies
Original post by Salmonidae
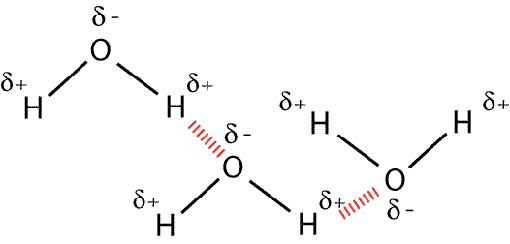
Hydrogen Bonds are a specific type of permanent dipole-permanent dipole bond where a delta-negatively charged Oxygen atom is attracted to a delta-positively charged Hydrogen atom (delta meaning small). Hydrogen is such a small atom that when these attractions form the distance between the hydrogen and the oxygen/nitrogen/fluorine atom (has to be one of those due to electronegative) it is covalently bonded to is almost the same as the distance between the hydrogen and the oxygen atom it is hydrogen bonded to. Therefore this type of intermolecular bond is exceptionally strong and we call it a hydrogen bond.
This diagram shows hydrogen bonding between water molecules
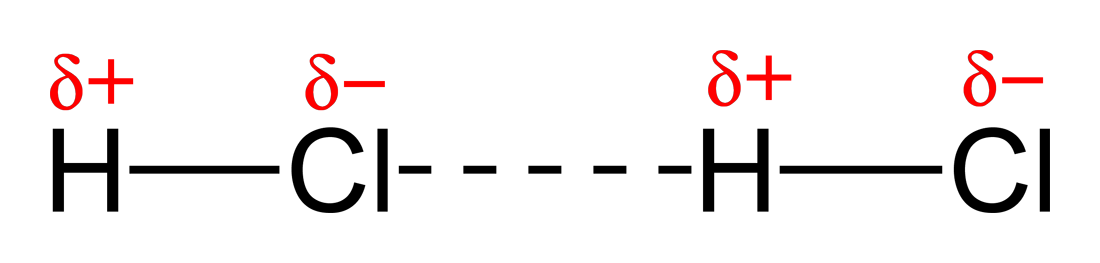
A permanent dipole-permanent dipole is formed exactly the same way as a hydrogen bond (i.e is the result of 2 polarised bonds) but can be with other atoms and is weaker due to bigger atoms involved and longer distances.
Here is a diagram showing permanent dipole-permanent dipole bonding in hydrogen chloride
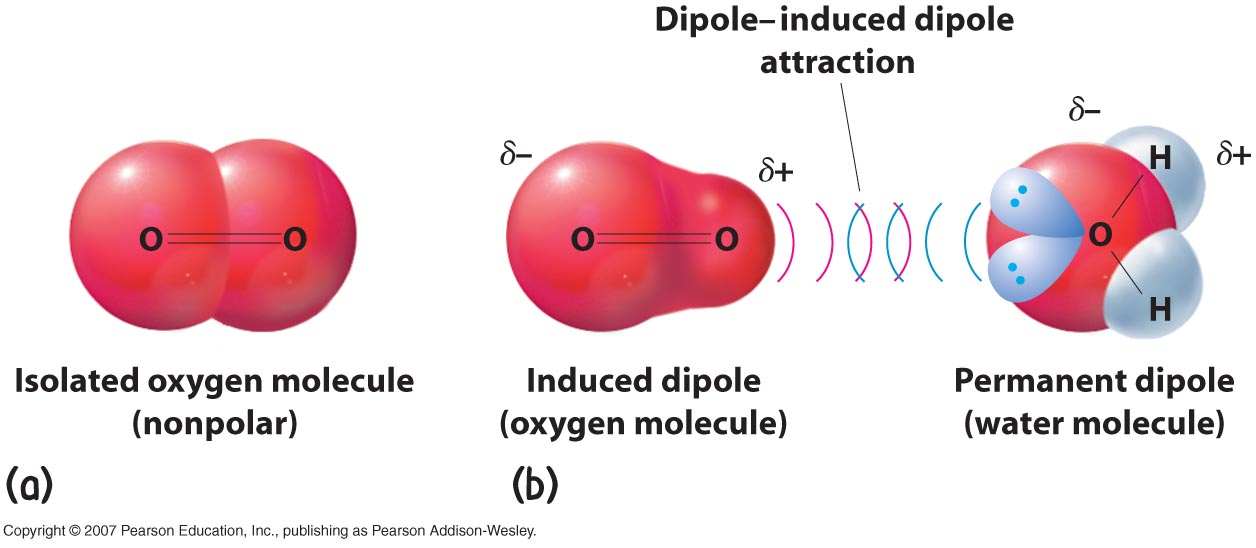
Permanent dipole - induced dipole is where a polarised bond (lets stick with H-Cl from the diagram above) induces (i.e creates) a dipole on another molecule and is therefore attracted to it. The works because if a negatively charged atom, i.e Cl in H-Cl, approaches a neutral atom the negative charge will distort the electron cloud on the neutral atom and push away the electrons creating a positive charge there. Therefore there will now be a permanent dipole-induced dipole attraction.
Here is a diagram showing water (a permanent dipole) inducing a dipole in an O2 molecule
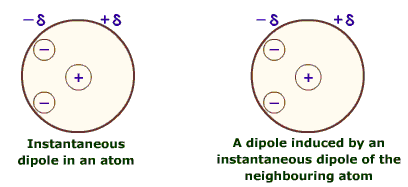
Finally an instantaneous dipole - induced dipole works in exactly the same way as a permanent dipole - induced dipole except that instead of a permanent dipole there is an instantaneous dipole. These arise randomly in any molecule and are caused by the random movement of electrons creating an imbalance of charge which then induces a dipole in the molecule next door and forming an intermolecular bond. This is the weakest intermolecular force, and is also known as Van der Waals forces.
Final diagram showing instantaneous dipole - induced dipole
Hope this helps, ask if you're still unsure!
S
This diagram shows hydrogen bonding between water molecules
A permanent dipole-permanent dipole is formed exactly the same way as a hydrogen bond (i.e is the result of 2 polarised bonds) but can be with other atoms and is weaker due to bigger atoms involved and longer distances.
Here is a diagram showing permanent dipole-permanent dipole bonding in hydrogen chloride
Permanent dipole - induced dipole is where a polarised bond (lets stick with H-Cl from the diagram above) induces (i.e creates) a dipole on another molecule and is therefore attracted to it. The works because if a negatively charged atom, i.e Cl in H-Cl, approaches a neutral atom the negative charge will distort the electron cloud on the neutral atom and push away the electrons creating a positive charge there. Therefore there will now be a permanent dipole-induced dipole attraction.
Here is a diagram showing water (a permanent dipole) inducing a dipole in an O2 molecule
Finally an instantaneous dipole - induced dipole works in exactly the same way as a permanent dipole - induced dipole except that instead of a permanent dipole there is an instantaneous dipole. These arise randomly in any molecule and are caused by the random movement of electrons creating an imbalance of charge which then induces a dipole in the molecule next door and forming an intermolecular bond. This is the weakest intermolecular force, and is also known as Van der Waals forces.
Final diagram showing instantaneous dipole - induced dipole
Hope this helps, ask if you're still unsure!
S
Thanks sooo much for that

thats seems to come up a lot and I never really learnt it properly. But you summarised it really well!
This was posted from The Student Room's Android App on my GT-I9100
Original post by 12sophie
Does anyone have a link to the JAN 2012 past paper and mark scheme please? x
It's been posted somewhere earlier on this thread.
Original post by JsConcernedMum
Sorry, concerned parent muscling in here, but hope you can help a bit please guys.
My son has literally just finished the course (last Friday) so not much real revision done as yet (Chemistry wasn't the only syllabus not finished! And he had three exams this week) He doesn't get study leave and has 4 other exams next week too, so time is veeeery short. Plus he has learning issues that slow him up!
(Chemistry wasn't the only syllabus not finished! And he had three exams this week) He doesn't get study leave and has 4 other exams next week too, so time is veeeery short. Plus he has learning issues that slow him up!
He's hoping to do this to degree level so it's pretty important to him.
I've read the thread, but are there any other tips you can suggest to make sure he spends his little time wisely please?
Zhy's comments on mark scheme answers sound like a great idea - J's pretty good at Chemistry, but lacks precision in his written answers so tends to drop marks on longer answers. I don't suppose anyone has summarised these and could let me have a copy please?
Any other thoughts gratefully received.
Thanks
My son has literally just finished the course (last Friday) so not much real revision done as yet
 (Chemistry wasn't the only syllabus not finished! And he had three exams this week) He doesn't get study leave and has 4 other exams next week too, so time is veeeery short. Plus he has learning issues that slow him up!
(Chemistry wasn't the only syllabus not finished! And he had three exams this week) He doesn't get study leave and has 4 other exams next week too, so time is veeeery short. Plus he has learning issues that slow him up!He's hoping to do this to degree level so it's pretty important to him.
I've read the thread, but are there any other tips you can suggest to make sure he spends his little time wisely please?
Zhy's comments on mark scheme answers sound like a great idea - J's pretty good at Chemistry, but lacks precision in his written answers so tends to drop marks on longer answers. I don't suppose anyone has summarised these and could let me have a copy please?

Any other thoughts gratefully received.
Thanks

I would recommend looking at the specification:
http://www.ocr.org.uk/download/kd/ocr_9605_kd_gce_spec.pdf
It's useful for long answer questions because it sometimes gives you exact answers they look for (e.g. global warming mechanism)

Original post by Salmonidae
Hey!
OK so the way displacement reactions work when it comes to the halogens is that the higher up elements displace the lower down ones. This is because the higher up elements are better oxidising agents, therefore they oxidise the other halogen and essentially 'steal' their electrons, becoming reduced themselves.
So in the case of your reactions:
Cl2 + 2KBr ---> Br2 + 2KCl This is because Cl is a better oxidising agent that Br so Br is oxidised and Cl is reduced, taking its place in the compound.
Cl2 + 2KI ----> I2 + 2KCL
Br2 + 2KI ----> I2 + 2KBr
Both these reactions work on the same principle.
Now as for colours in cyclohexane you just have to see which halogen is oxidised for it is this one which will dissolve in the cyclohexane.
Chlorine in cyclohexane is virtually colourless
Bromine in cyclohexane is an orange/red colour.
Iodine in cyclohexane is a pink/violet colour.
From this information you should be able to figure out that the first equation Bromine is oxidised, so the cyclohexane would be orange/red, and the 2nd and 3rd equations Iodine is oxidised so the cyclohexane would be pink/violet.
Hope this helps
S
OK so the way displacement reactions work when it comes to the halogens is that the higher up elements displace the lower down ones. This is because the higher up elements are better oxidising agents, therefore they oxidise the other halogen and essentially 'steal' their electrons, becoming reduced themselves.
So in the case of your reactions:
Cl2 + 2KBr ---> Br2 + 2KCl This is because Cl is a better oxidising agent that Br so Br is oxidised and Cl is reduced, taking its place in the compound.
Cl2 + 2KI ----> I2 + 2KCL
Br2 + 2KI ----> I2 + 2KBr
Both these reactions work on the same principle.
Now as for colours in cyclohexane you just have to see which halogen is oxidised for it is this one which will dissolve in the cyclohexane.
Chlorine in cyclohexane is virtually colourless
Bromine in cyclohexane is an orange/red colour.
Iodine in cyclohexane is a pink/violet colour.
From this information you should be able to figure out that the first equation Bromine is oxidised, so the cyclohexane would be orange/red, and the 2nd and 3rd equations Iodine is oxidised so the cyclohexane would be pink/violet.
Hope this helps
S
thats GREAT thank you . xx
Original post by 12sophie
Does anyone have a link to the JAN 2012 past paper and mark scheme please? x
We can't get access to the paper but teachers should be -- mine gave me it. Send an email to the chem teacher.
 if they can't send it I could try attaching the Q's.
if they can't send it I could try attaching the Q's.Original post by fallen_child
what is the best advices to tackle this paper???
Revision

Original post by 12sophie
Does anyone have a link to the JAN 2012 past paper and mark scheme please? x

can someone explain to me how i would work out the formula of calcium chlorate(I)?
Original post by Kreayshawn
can someone explain to me how i would work out the formula of calcium chlorate(I)?
The formula is Ca(ClO3)2 and the best way to remember it would just to remember that the chlorate ion (ClO3-1) has a negative 1 charge. However this is hardly a common ion and I doubt it would come up.
Original post by Salmonidae
The formula is Ca(ClO3)2 and the best way to remember it would just to remember that the chlorate ion (ClO3-1) has a negative 1 charge. However this is hardly a common ion and I doubt it would come up.
mark scheme says Ca(ClO)2 and i'm not entirely sure how they got there
Original post by Kreayshawn
mark scheme says Ca(ClO)2 and i'm not entirely sure how they got there
How could that possibly right? The oxygen would have to form some sort of dative double bond with the chloride....
Well either way the internet disagrees. Which paper and question is this?
Original post by Kreayshawn
mark scheme says Ca(ClO)2 and i'm not entirely sure how they got there
It is Ca(ClO)2. Since the oxidation state of Cl is +1, the chlorate(I) ion is ClO-. The Ca ion is 2+ so the formula must be Ca(ClO)2.
(edited 11 years ago)
Original post by Zhy
It is Ca(ClO)2. Since the oxidation state of Cl is +1, the chlorate ion is ClO-. The Ca ion is 2+ so the formula must be Ca(ClO)2.
Ah right I see, just interesting that if you google calcium chlorate you get a completely different compound.
Original post by Salmonidae
How could that possibly right? The oxygen would have to form some sort of dative double bond with the chloride....
Well either way the internet disagrees. Which paper and question is this?
Well either way the internet disagrees. Which paper and question is this?
june 2011, question 1) b) i)
Original post by Zhy
It is Ca(ClO)2. Since the oxidation state of Cl is +1, the chlorate ion is ClO-. The Ca ion is 2+ so the formula must be Ca(ClO)2.
thanks, that makes sense
for these types of questions where it asks you to show the overall equation for the reaction, what do you do?
equation 2.1: NO(g) + ½ O2(g) --> NO2(g)
equation 2.2: NO2(g) --> NO(g) + O(g)
equation 2.3: O2(g) + O(g) --> O3(g)
i don't understand how the overall equation ends up being 1½ O2 --> O3, since you start with just ½ O2
(edited 11 years ago)
Original post by Salmonidae
Ah right I see, just interesting that if you google calcium chlorate you get a completely different compound.
Thats because the chlorine exists in many oxidation states.
Original post by Kreayshawn
for these types of questions where it asks you to show the overall equation for the reaction, what do you do?
equation 2.1: NO(g) + ½ O2(g) --> NO2(g)
equation 2.2: NO2(g) --> NO(g) + O(g)
equation 2.3: O2(g) + O(g) --> O3(g)
i don't understand how the overall equation ends up being 1½ O2 --> O3, since you start with just ½ O2
for these types of questions where it asks you to show the overall equation for the reaction, what do you do?
equation 2.1: NO(g) + ½ O2(g) --> NO2(g)
equation 2.2: NO2(g) --> NO(g) + O(g)
equation 2.3: O2(g) + O(g) --> O3(g)
i don't understand how the overall equation ends up being 1½ O2 --> O3, since you start with just ½ O2
Ok this is a little bit tricky to get your head around.
If we examine the equations:
equation 2.1: NO(g) + ½ O2(g) --> NO2(g)
equation 2.2: NO2(g) --> NO(g) + O(g)
Here you can see quite plainly that NO is just catalysing the breakdown of O2 into O, the ½ has just been used in order to make it balanced without lots of numbers everywhere.
So therefore if you cancel out the catalyst (as it is unchanged) you end up with
½ O2(g) --> O(g)
The final equation:
equation 2.3: O2(g) + O(g) --> O3(g)
Is just showing how an O2 from the atmosphere can react with the O we just made in order to create ozone.
The answer to this question is simply found by combining:
½O2(g) --> O(g)
and
O2(g) + O(g) --> O3(g)
and by assuming the top equation of O2 to O happens spontaneously that leaves us:
1½O2 ---> O3
Hope that makes some more sense
S
PS
Original post by ManPowa
Thats because the chlorine exists in many oxidation states.
Yes I understand that now, was just confused because the question was proposed out of context.

(edited 11 years ago)
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- A Level Exam Discussions 2023
- OCR Chemistry B (Salters) - Anybody doing this?
- GCSE Exam Discussions 2024
- GCSE Exam Discussions 2023
- SQA Nat 5 Chemistry - 23rd May 2024 [Exam Chat]
- Is it possible to change sixth forms now (in year 12)?
- SQA Higher Chemistry - Paper 2 - 23rd May 2024 [Exam Chat]
- Revision website for OCR B (salters) chemistry
- AS and A level urgent help & advice
- chemistry
- Edexcel chemistry unit 2 mixed questions
- OCR A-Level Chemistry B Paper 3 (H433/03) - 23rd June 2023 [Exam Chat]
- A Level Chemistry (OCR B Salters Chemistry)
- AS/A Level Chemistry Study Group 2023/2024
- OCR A Level Business Paper 1 (H431/01) - 23rd May 2023 [Exam Chat]
- How to get A/A* in A level chemistry
- KU Cost of Living Support: Student Pantry
- OCR AS-Level History Unit 2 (Y243,Y249,Y251-253) - 23rd May 2023 [Exam Chat]
- A Level Chemistry Mark Scheme
Latest
Last reply 2 minutes ago
The Official King's College London Applicants for 2024 Entry ThreadLast reply 2 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 4 minutes ago
Goldmansachs Degree Apprenticeship 2024Last reply 7 minutes ago
What does a timetable look like for a psychology student at the UOL?Last reply 8 minutes ago
Official: Aston University A100 2024 Entry Applicant threadMedical Schools
1161
Last reply 8 minutes ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1314
Last reply 10 minutes ago
AQA GCSE Religious Studies Paper 1 (8062/ 11-17) - 15th May 2023 [Exam Chat]Last reply 11 minutes ago
India Willoughby reports jk rowling to the policeLast reply 11 minutes ago
AQA A Level Spanish Paper 3 (Speaking/IRP) 7692/3 - 2024 [Exam Chat]Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]




