adding NaOH onto a polyester
QUOTED FROM CHEMGUIDE:
Hydrolysis of polyesters
Simple esters are easily hydrolysed by reaction with dilute acids or alkalis.
Polyesters are attacked readily by alkalis, but much more slowly by dilute acids. Hydrolysis by water alone is so slow as to be completely unimportant. (You wouldn't expect your polyester fleece to fall to pieces if you went out in the rain!)
If you spill dilute alkali on a fabric made from polyester, the ester linkages are broken. Ethane-1,2-diol is formed together with the salt of the carboxylic acid.
Because you produce small molecules rather than the original polymer, the fibres are destroyed, and you end up with a hole!
For example, if you react the polyester with sodium hydroxide solution:
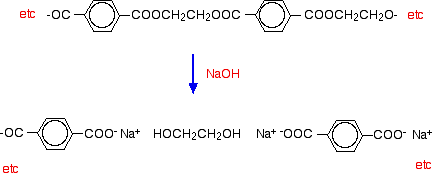
It says that spilling a dilute alkali will separate the ester link.
However I have come across a question which asks "if you add hot concentrated aqueous sodium hydroxide"
In this case, would the ester linkages break?
I think it doesn't..... but could someone confirm and give a reasoning?
Hydrolysis of polyesters
Simple esters are easily hydrolysed by reaction with dilute acids or alkalis.
Polyesters are attacked readily by alkalis, but much more slowly by dilute acids. Hydrolysis by water alone is so slow as to be completely unimportant. (You wouldn't expect your polyester fleece to fall to pieces if you went out in the rain!)
If you spill dilute alkali on a fabric made from polyester, the ester linkages are broken. Ethane-1,2-diol is formed together with the salt of the carboxylic acid.
Because you produce small molecules rather than the original polymer, the fibres are destroyed, and you end up with a hole!
For example, if you react the polyester with sodium hydroxide solution:

It says that spilling a dilute alkali will separate the ester link.
However I have come across a question which asks "if you add hot concentrated aqueous sodium hydroxide"
In this case, would the ester linkages break?
I think it doesn't..... but could someone confirm and give a reasoning?
Generally, if a dilute solution will react then a concentrated one will react faster.
Original post by EierVonSatan
Generally, if a dilute solution will react then a concentrated one will react faster.
"concentrated will attack the polyester too slowly to be a problem"
I quoted this from a mark scheme.... what does this mean?
Original post by LiAlH4
"concentrated will attack the polyester too slowly to be a problem"
I quoted this from a mark scheme.... what does this mean?
I quoted this from a mark scheme.... what does this mean?
I don't know, it's not a great sentence. I've been looking around to find evidence that higher concentration of base is detrimental to rate of hydrolysis, but haven't seen anything concrete.
I'm quite confident that if I placed a polyester shirt in a hot bath of concentrated sodium hydroxide it wouldn't survive the experience.
(edited 10 years ago)
Quick Reply
Related discussions
- chemistry question
- AQA AS Chemistry 2017 Paper 1 Titration MCQ Q16
- Chemistry Moles
- Chemistry a level buffers questions
- [Chemistry] Equilibrium Question
- chem help
- Acids and Bases
- Chemical calculations
- Hard A Level Chemistry Question
- titration... moley moles...
- urgent help.
- Back-titration question
- AS Organic Chem Practical Question
- Kc Titration Question
- chemistry alevel
- A level Chemistry Titration
- Tollens question
- a level chemistry
- Dilution calculations
- hard titration alevel chem Q!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products




