How does a scanning tunnelling microscope work?
Just assume I know nothing about quantum tunnelling! Which is not far from the truth anyway...
What does the pd between the tip and surface do?
Where do the electrons go when they reach the tip?
Where does the electrostatic barrier arise from?
Is there is a voltage between the surface and tip that attracts the electrons toward the tip, how can this be a 'barrier'?
Its undergrad stuff heavily watered down for the optional 'turning points' module in A2, leaving many gaps unfilled imo
Thanks!
Posted from TSR Mobile
What does the pd between the tip and surface do?
Where do the electrons go when they reach the tip?
Where does the electrostatic barrier arise from?
Is there is a voltage between the surface and tip that attracts the electrons toward the tip, how can this be a 'barrier'?
Its undergrad stuff heavily watered down for the optional 'turning points' module in A2, leaving many gaps unfilled imo
Thanks!
Posted from TSR Mobile
Original post by tmorrall
.....
For any of the 'advanced' stuff they present to you at A-level, I would suggest you just memorise it for the exam and move swiftly on. Some of the topics they cover are almost impossible to explain without a stronger understanding in areas of maths and physics.
To keep it simple, I'll try to cover it in these images:

This is a model of a typical 'particle in a box' thought-experiment. Focus on models B, C and D because they're easier to understand. They're standing waves, which I think you should know about from the A-level syllabus. The walls of this box are not physical walls, but are barriers of infinite potential. For example, imagine a charged particle existing between two instantaneous electric fields that are impossible to push through, so that the particle would be forever confined to the space in between.
For this to be the case, and assuming the De Broglie hypothesis of all particles exhibiting wave-like properties, the particle wave must be such that its amplitude is zero at the edges of the box. Otherwise its reflection at the boundary would be somewhat chaotic. The same as standing waves.
However. See next image:
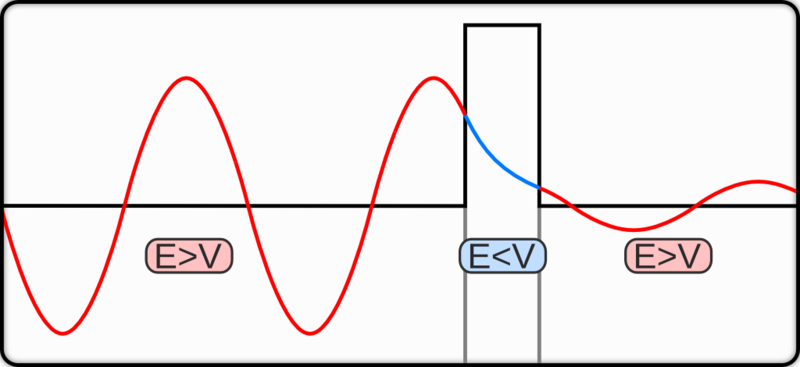
The reality of the barrier of potential is that it is not ever going to be truly infinite, and so some particles, given the correct circumstances, will be able to surpass the barrier. There are certain probabilistic aspects involved which I am yet to be taught myself, but that shouldn't really be a problem in explaining this.
The process of particles crossing a barrier like this is called quantum tunnelling.
Since the particle has passed the barrier, it will then exist in a new space and can be detected by some device that, when the particle is incident on it, will induce some small current.
By scanning across the surface of a material, some electrons will be close enough, or configured rightly enough, to escape the potential barrier and jump across to be detected by the device.
With all that in mind, hopefully this final description will make good sense:

Original post by Pessimisterious
For any of the 'advanced' stuff they present to you at A-level, I would suggest you just memorise it for the exam and move swiftly on. Some of the topics they cover are almost impossible to explain without a stronger understanding in areas of maths and physics.
To keep it simple, I'll try to cover it in these images:

This is a model of a typical 'particle in a box' thought-experiment. Focus on models B, C and D because they're easier to understand. They're standing waves, which I think you should know about from the A-level syllabus. The walls of this box are not physical walls, but are barriers of infinite potential. For example, imagine a charged particle existing between two instantaneous electric fields that are impossible to push through, so that the particle would be forever confined to the space in between.
For this to be the case, and assuming the De Broglie hypothesis of all particles exhibiting wave-like properties, the particle wave must be such that its amplitude is zero at the edges of the box. Otherwise its reflection at the boundary would be somewhat chaotic. The same as standing waves.
However. See next image:

The reality of the barrier of potential is that it is not ever going to be truly infinite, and so some particles, given the correct circumstances, will be able to surpass the barrier. There are certain probabilistic aspects involved which I am yet to be taught myself, but that shouldn't really be a problem in explaining this.
The process of particles crossing a barrier like this is called quantum tunnelling.
Since the particle has passed the barrier, it will then exist in a new space and can be detected by some device that, when the particle is incident on it, will induce some small current.
By scanning across the surface of a material, some electrons will be close enough, or configured rightly enough, to escape the potential barrier and jump across to be detected by the device.
With all that in mind, hopefully this final description will make good sense:

To keep it simple, I'll try to cover it in these images:

This is a model of a typical 'particle in a box' thought-experiment. Focus on models B, C and D because they're easier to understand. They're standing waves, which I think you should know about from the A-level syllabus. The walls of this box are not physical walls, but are barriers of infinite potential. For example, imagine a charged particle existing between two instantaneous electric fields that are impossible to push through, so that the particle would be forever confined to the space in between.
For this to be the case, and assuming the De Broglie hypothesis of all particles exhibiting wave-like properties, the particle wave must be such that its amplitude is zero at the edges of the box. Otherwise its reflection at the boundary would be somewhat chaotic. The same as standing waves.
However. See next image:

The reality of the barrier of potential is that it is not ever going to be truly infinite, and so some particles, given the correct circumstances, will be able to surpass the barrier. There are certain probabilistic aspects involved which I am yet to be taught myself, but that shouldn't really be a problem in explaining this.
The process of particles crossing a barrier like this is called quantum tunnelling.
Since the particle has passed the barrier, it will then exist in a new space and can be detected by some device that, when the particle is incident on it, will induce some small current.
By scanning across the surface of a material, some electrons will be close enough, or configured rightly enough, to escape the potential barrier and jump across to be detected by the device.
With all that in mind, hopefully this final description will make good sense:

Great explanation! So the electron can exist wherever its wavelength extends to? So are these 'barriers' the charge difference and the tip and the surface? Thanks thats really clearing things up.
Posted from TSR Mobile
Original post by tmorrall
Great explanation! So the electron can exist wherever its wavelength extends to? So are these 'barriers' the charge difference and the tip and the surface? Thanks thats really clearing things up.
Posted from TSR Mobile
Posted from TSR Mobile
In the case of the scanning microscope, I'm actually not sure what the barrier really is. My best guess would be that the barrier is the energy level that the electron exists within. Just like the photoelectric effect only happens for light of a high enough energy, the electron tunnelling probably only happens when the voltage of the electron microscope is high enough to pull electrons out. It may be related to the whole E=hf thing. (I could be totally wrong with that assumption though).
In the images in my previous post, the waves didn't actually physically represent the electron. They're probability waves. It's essentially a wave 'image' from which you can attempt to deduce everything else about the particle. So it's not that the electron's wavelength 'extends', but it's probability amplitude is such that it has a chance of existing beyond the confines of the energy barrier. There's a very real chance that some electrons will go beyond the confines of the energy well, and this is what the microscope relies on. This can only happen for cases where the electron energy is high enough, or if the energy of the barrier is such that it can push past it.
(edited 9 years ago)
Original post by Pessimisterious
In the case of the scanning microscope, I'm actually not sure what the barrier really is. My best guess would be that the barrier is the energy level that the electron exists within. Just like the photoelectric effect only happens for light of a high enough energy, the electron tunnelling probably only happens when the voltage of the electron microscope is high enough to pull electrons out. It may be related to the whole E=hf thing. (I could be totally wrong with that assumption though).
In the images in my previous post, the waves didn't actually physically represent the electron. They're probability waves. It's essentially a wave 'image' from which you can attempt to deduce everything else about the particle. So it's not that the electron's wavelength 'extends', but it's probability amplitude is such that it has a chance of existing beyond the confines of the energy barrier. There's a very real chance that some electrons will go beyond the confines of the energy well, and this is what the microscope relies on. This can only happen for cases where the electron energy is high enough, or if the energy of the barrier is such that it can push past it.
In the images in my previous post, the waves didn't actually physically represent the electron. They're probability waves. It's essentially a wave 'image' from which you can attempt to deduce everything else about the particle. So it's not that the electron's wavelength 'extends', but it's probability amplitude is such that it has a chance of existing beyond the confines of the energy barrier. There's a very real chance that some electrons will go beyond the confines of the energy well, and this is what the microscope relies on. This can only happen for cases where the electron energy is high enough, or if the energy of the barrier is such that it can push past it.
Is the barrier not just the "gap" between the probe and the surface? When it is of the order of about 1nm (de broglie wavelength of electrons in a metal at room temp) there is a finite probability the electrons will tunnel across the gap (as long as it is sufficiently small)
The probe is at a constant -1 V voltage so that electrons only tunnel 1 way across the gap (probe to surface) otherwise electrons would tunnel both ways and there would be no net flow of current to monitor.
(edited 9 years ago)
Original post by Davelittle
Is the barrier not just the "gap" between the probe and the surface? When it is of the order of about 1nm (de broglie wavelength of electrons in a metal at room temp) there is a finite probability the electrons will tunnel across the gap (as long as it is sufficiently small)
The probe is at a constant -1 V voltage so that electrons only tunnel 1 way across the gap (probe to surface) otherwise electrons would tunnel both ways and there would be no net flow of current to monitor.
The probe is at a constant -1 V voltage so that electrons only tunnel 1 way across the gap (probe to surface) otherwise electrons would tunnel both ways and there would be no net flow of current to monitor.
Yeah I think the barrier is partly defined by the size of the gap between the device and the surface.
I don't think there would be a problem with them tunnelling both ways though. Regardless of which ways the electrons travel, a small current would still be detected, and that's enough to make up the desired image. Because all the microscope actually does is create an image based on the amount of current formed for certain voltages and certain heights above the surface.
e.g. at one position there are X volts recorded, and at another position there are X fewer volts recorded, therefore the surface of the material must be at a different height there. Which is presumably then mapped by the software connected to the microscope.
Original post by Pessimisterious
Yeah I think the barrier is partly defined by the size of the gap between the device and the surface.
I don't think there would be a problem with them tunnelling both ways though. Regardless of which ways the electrons travel, a small current would still be detected, and that's enough to make up the desired image. Because all the microscope actually does is create an image based on the amount of current formed for certain voltages and certain heights above the surface.
e.g. at one position there are X volts recorded, and at another position there are X fewer volts recorded, therefore the surface of the material must be at a different height there. Which is presumably then mapped by the software connected to the microscope.
I don't think there would be a problem with them tunnelling both ways though. Regardless of which ways the electrons travel, a small current would still be detected, and that's enough to make up the desired image. Because all the microscope actually does is create an image based on the amount of current formed for certain voltages and certain heights above the surface.
e.g. at one position there are X volts recorded, and at another position there are X fewer volts recorded, therefore the surface of the material must be at a different height there. Which is presumably then mapped by the software connected to the microscope.
Ah ok, my book says about the tunnelling both ways thing to explain the -ve p.d. of the tip but that may be a simplified explanation!
Original post by Davelittle
Ah ok, my book says about the tunnelling both ways thing to explain the -ve p.d. of the tip but that may be a simplified explanation!
Hmm well your book probably explains it better than I can! Haha. I could be quite wrong with the stuff I said. I'm just kind of trying to logically put two and two together, based on what I've learned so far.
I think I am mixing up de Broglie wavelength and the probability function which I don't suppose you can connect?
I see what you're both saying. I think the PD is to keep as many electrons as possible flowing surface to tip right? Without the PD there would be equal probability of the existing anywhere other than the tip as well as the tip but with a charge bias any electron tunnelling from the surface to the region between probe and surface will be attracted to the tip. Without a pd surely there is no reason a current should flow and create the image?!
Appreciate the help guys!
Posted from TSR Mobile
Original post by Davelittle
Ah ok, my book says about the tunnelling both ways thing to explain the -ve p.d. of the tip but that may be a simplified explanation!
Original post by Pessimisterious
Hmm well your book probably explains it better than I can! Haha. I could be quite wrong with the stuff I said. I'm just kind of trying to logically put two and two together, based on what I've learned so far.
I see what you're both saying. I think the PD is to keep as many electrons as possible flowing surface to tip right? Without the PD there would be equal probability of the existing anywhere other than the tip as well as the tip but with a charge bias any electron tunnelling from the surface to the region between probe and surface will be attracted to the tip. Without a pd surely there is no reason a current should flow and create the image?!
Appreciate the help guys!
Posted from TSR Mobile
Original post by tmorrall
I think I am mixing up de Broglie wavelength and the probability function which I don't suppose you can connect?
I see what you're both saying. I think the PD is to keep as many electrons as possible flowing surface to tip right? Without the PD there would be equal probability of the existing anywhere other than the tip as well as the tip but with a charge bias any electron tunnelling from the surface to the region between probe and surface will be attracted to the tip. Without a pd surely there is no reason a current should flow and create the image?!
Appreciate the help guys!
Posted from TSR Mobile
I see what you're both saying. I think the PD is to keep as many electrons as possible flowing surface to tip right? Without the PD there would be equal probability of the existing anywhere other than the tip as well as the tip but with a charge bias any electron tunnelling from the surface to the region between probe and surface will be attracted to the tip. Without a pd surely there is no reason a current should flow and create the image?!
Appreciate the help guys!
Posted from TSR Mobile
Yeah the de Broglie wavelength is quite a far way off from the wave function.
You can find the de Broglie wavelength by using the momentum operator on the function and taking it from there, but... generally the connection is probably not what you're thinking.
The typical image of a wave function in quantum physics is not at all supposed to represent the actual particle as a wave. It's only called a wave function because it is, mathematically speaking, an actual wave function. What it really means is that the solution to the Schrodinger equation must be a wave function because a wave function is the only thing that will satisfy it.
The Shrodinger equation is just a nifty version of this:
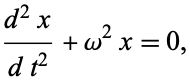
which you'll find can only really be satisfied by functions that are considered waves. For example, if you try x=sin(wx), it'll be valid, and so the solution is a wave function. The same goes for the Shrodinger equation. The solution is a wave function, but the graphical visualisation of the wave function doesn't really bare much importance on the way the particle will actually 'appear' to be. You need to mathematically manipulate the function a bit to draw any other specific info from it.
Hmmm ... about the PD... It's actually nothing special really. The PD is just a PD, and the microscope simply makes use of the phenomenon of quantum tunnelling to get readings.
Essentially it provides an attractive electric field that draws electrons towards it. However the attractive force drops off with 1/r2, (Coulomb Law). So any differences in surface height across the material will mean the attractive force is higher or lower. When the attractive force is higher, more electrons jump (tunnel) past the potential energy barrier that was confining them. The microscope then reads a higher current at these parts, and lower currents at others parts, which is all then mapped into an image to show the surface in 3D.
Hope that makes some sense!
Oh and yeah I guess the PD does increase the probability of the electron existing outside of its original confines, as shown in the second image of my original reply.
Original post by Pessimisterious
Yeah the de Broglie wavelength is quite a far way off from the wave function.
You can find the de Broglie wavelength by using the momentum operator on the function and taking it from there, but... generally the connection is probably not what you're thinking.
The typical image of a wave function in quantum physics is not at all supposed to represent the actual particle as a wave. It's only called a wave function because it is, mathematically speaking, an actual wave function. What it really means is that the solution to the Schrodinger equation must be a wave function because a wave function is the only thing that will satisfy it.
The Shrodinger equation is just a nifty version of this:
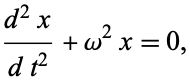
which you'll find can only really be satisfied by functions that are considered waves. For example, if you try x=sin(wx), it'll be valid, and so the solution is a wave function. The same goes for the Shrodinger equation. The solution is a wave function, but the graphical visualisation of the wave function doesn't really bare much importance on the way the particle will actually 'appear' to be. You need to mathematically manipulate the function a bit to draw any other specific info from it.
Hmmm ... about the PD... It's actually nothing special really. The PD is just a PD, and the microscope simply makes use of the phenomenon of quantum tunnelling to get readings.
Essentially it provides an attractive electric field that draws electrons towards it. However the attractive force drops off with 1/r2, (Coulomb Law). So any differences in surface height across the material will mean the attractive force is higher or lower. When the attractive force is higher, more electrons jump (tunnel) past the potential energy barrier that was confining them. The microscope then reads a higher current at these parts, and lower currents at others parts, which is all then mapped into an image to show the surface in 3D.
Hope that makes some sense!
Oh and yeah I guess the PD does increase the probability of the electron existing outside of its original confines, as shown in the second image of my original reply.
You can find the de Broglie wavelength by using the momentum operator on the function and taking it from there, but... generally the connection is probably not what you're thinking.
The typical image of a wave function in quantum physics is not at all supposed to represent the actual particle as a wave. It's only called a wave function because it is, mathematically speaking, an actual wave function. What it really means is that the solution to the Schrodinger equation must be a wave function because a wave function is the only thing that will satisfy it.
The Shrodinger equation is just a nifty version of this:
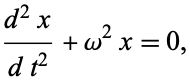
which you'll find can only really be satisfied by functions that are considered waves. For example, if you try x=sin(wx), it'll be valid, and so the solution is a wave function. The same goes for the Shrodinger equation. The solution is a wave function, but the graphical visualisation of the wave function doesn't really bare much importance on the way the particle will actually 'appear' to be. You need to mathematically manipulate the function a bit to draw any other specific info from it.
Hmmm ... about the PD... It's actually nothing special really. The PD is just a PD, and the microscope simply makes use of the phenomenon of quantum tunnelling to get readings.
Essentially it provides an attractive electric field that draws electrons towards it. However the attractive force drops off with 1/r2, (Coulomb Law). So any differences in surface height across the material will mean the attractive force is higher or lower. When the attractive force is higher, more electrons jump (tunnel) past the potential energy barrier that was confining them. The microscope then reads a higher current at these parts, and lower currents at others parts, which is all then mapped into an image to show the surface in 3D.
Hope that makes some sense!
Oh and yeah I guess the PD does increase the probability of the electron existing outside of its original confines, as shown in the second image of my original reply.
Nice little bit of undergrad for me to sink my teeth into, shame I'm hopefully doing engineering!
A little bit more never hurt!
Cheers for all the help again
Posted from TSR Mobile
Original post by tmorrall
Nice little bit of undergrad for me to sink my teeth into, shame I'm hopefully doing engineering!
A little bit more never hurt!
Cheers for all the help again
Posted from TSR Mobile
A little bit more never hurt!
Cheers for all the help again
Posted from TSR Mobile
Heh, there's a chance you'll see that basic equation anyway though!
You've done simple harmonic motion yet?
The equation simply represents any simple harmonic oscillator.
Linky
Anyway, good luck!
Original post by Pessimisterious
Heh, there's a chance you'll see that basic equation anyway though!
You've done simple harmonic motion yet?
The equation simply represents any simple harmonic oscillator.
Linky
Anyway, good luck!
You've done simple harmonic motion yet?
The equation simply represents any simple harmonic oscillator.
Linky
Anyway, good luck!
oh yeah should have spotted that the second differential of displacement is equal to the negative angular frequency squared by displacement right? I will actually have to look at how that ties in now. Thanks for the procrastinating material

Quick Reply
Related discussions
- A-level Biology Question (AQA)
- Can laser scanning confocal microcsopes produce images if the specimen isnt tagged wi
- mark scheme biology 2022 gcse combined science higher paper 1
- Using gel pens in exams?
- AQA GCSE Biology Paper 1 (Higher Tier) 8461/1H - 17 May 2022 [Unofficial Mark Scheme]
- Can one swim to the euro tunnel?
- homework help
- LNAT Palm Vein Scan
- CIE Biology A Level
- how to fix this after fasting requires blackout or fainting
- Do all light microscpes only view living cells?
- Help I’ve used a frixion pen on my sqa exam
- Biology
- Gcse coursework
- diagnostic radiography epq ideas
- Gcse corse work
- Medical Degree Doctor Apprenticeship
- John Lewis Partner Discount
- Tesco Right to Work Check Issue
- Can anyone please help with this maths question
Latest
Last reply 7 minutes ago
Got Red Light Penalty Notice - First Time Offender - Course EligibilityLast reply 13 minutes ago
Official Newcastle University Offer Holders Thread for 2024 entryLast reply 17 minutes ago
Zoom to Zoology? Uni open days, applications & more 2021-Present - a parents takeLast reply 25 minutes ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadLast reply 32 minutes ago
2024 entry A100 / A101 Medicine fastest and slowest offer sendersMedicine
852
Last reply 36 minutes ago
The Official Funding questions/moans/possible joy ThreadPosted 37 minutes ago
Student Finance applications for Part Time studentsPosted 41 minutes ago
Better Uni for Master decree in Business SustainalibityLast reply 42 minutes ago
I want to quit physio, maybe to start a career in IT



