AS Chemistry- helping each other out!
Scroll to see replies
Original post by lucye7
hi! Sorry if i've done this wrong this is my first post! I'm doing AQA AS Chem and the spec says "appreciate the usefulness of these reactions (nucleophillic substitution) in organic synthesis" but i don't really have any specific examples and was wondering if someone could help me out  thank youuuu
thank youuuu
 thank youuuu
thank youuuuHi there!
You haven't done anything wrong

Uh in the exam they won't ask you to name an example of a nucleophilic substitution reaction.
What they will do is show you a chemical reaction and they'll ask you to name and outline a mechanism for it.
Here's an example:
And then this what you do:
Questions like this will usually be 5 marks but it can be 6 marks if they ask you to name the product.
I hope that helped cleared some things up

Original post by kandykissesxox
Hi guys this may be a really stupid question but I was wondering why is CCl2 isn't the BENT shape so wouldn't the angle be 104.5 if it has one lone pair? I'm really confused not sure where 118 degrees has come from
Hi guys this may be a really stupid question but I was wondering why is CCl2 isn't the BENT shape so wouldn't the angle be 104.5 if it has one lone pair? I'm really confused not sure where 118 degrees has come from
Posted from TSR Mobile
CCL2 has 2 bonding pairs and a lone pair,so it's non linear with a bond angle of 104.5 degrees.
Original post by kandykissesxox
Hi guys this may be a really stupid question but I was wondering why is CCl2 isn't the BENT shape so wouldn't the angle be 104.5 if it has one lone pair? I'm really confused not sure where 118 degrees has come from
Hi guys this may be a really stupid question but I was wondering why is CCl2 isn't the BENT shape so wouldn't the angle be 104.5 if it has one lone pair? I'm really confused not sure where 118 degrees has come from
If you draw the dot and cross diagram for CCl2 and, say, H2O, you see that because carbon only has 4 outer electrons 2 join in the bonding which leaves 2 electrons - ie 1 lone pair. In H2O you have 4 electrons left, so 2 lone pairs. This means that in CCl2 it is trigonal planar but with the lone pair pushing the bond angle out.
Original post by Kadak
Posted from TSR Mobile
CCL2 has 2 bonding pairs and a lone pair,so it's non linear with a bond angle of 104.5 degrees.
CCL2 has 2 bonding pairs and a lone pair,so it's non linear with a bond angle of 104.5 degrees.
but where has the 118 come from?
Original post by ThatPerson2
If you draw the dot and cross diagram for CCl2 and, say, H2O, you see that because carbon only has 4 outer electrons 2 join in the bonding which leaves 2 electrons - ie 1 lone pair. In H2O you have 4 electrons left, so 2 lone pairs. This means that in CCl2 it is trigonal planar but with the lone pair pushing the bond angle out.
i thought it was a bent shape?
Can someones give me the definition of bond angle & bond length (edexcel)
Original post by kandykissesxox
Hi guys this may be a really stupid question but I was wondering why is CCl2 isn't the BENT shape so wouldn't the angle be 104.5 if it has one lone pair? I'm really confused not sure where 118 degrees has come from
Hi guys this may be a really stupid question but I was wondering why is CCl2 isn't the BENT shape so wouldn't the angle be 104.5 if it has one lone pair? I'm really confused not sure where 118 degrees has come from
Bent shapes are for molecules that have two bonding pairs and two lone pairs like H20.
*Please note that that diagram should say 104.5o
CCl2 has two bonding pairs and one lone pair of electrons.
As we know for every lone pair added to a molecule the bond angle is reduced 2.5o.
So in this case we should reduce 120o (angle for 3 bonding pairs) by 2.5o because a lone pair is added to the structure of CCl2.
I hope that helps

Original post by Disney0702
Bent shapes are for molecules that have two bonding pairs and two lone pairs like H20.
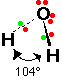
*Please note that that diagram should say 104.5o
CCl2 has two bonding pairs and one lone pair of electrons.
As we know for every lone pair added to a molecule the bond angle is reduced 2.5o.
So in this case we should reduce 120o (angle for 3 bonding pairs) by 2.5o because a lone pair is added to the structure of CCl2.
I hope that helps
*Please note that that diagram should say 104.5o
CCl2 has two bonding pairs and one lone pair of electrons.
As we know for every lone pair added to a molecule the bond angle is reduced 2.5o.
So in this case we should reduce 120o (angle for 3 bonding pairs) by 2.5o because a lone pair is added to the structure of CCl2.
I hope that helps

Ohh thank you so much! this made a lot of sense

Guys, are we meant to know how mass spectrometry works? (FOR OCR A) If so, can anyone break it down for me?
Original post by kandykissesxox
Ohh thank you so much! this made a lot of sense 

Oh you're welcome, I'm glad you found that useful.
May I ask what exam board you are and how old that question is?
Original post by kandykissesxox
but where has the 118 come from?
There's a little rule where you add 2.5 degrees per lone pair.
Original post by Disney0702
Oh you're welcome, I'm glad you found that useful.
May I ask what exam board you are and how old that question is?
May I ask what exam board you are and how old that question is?
It's AQA and the past paper was from 2002

Original post by Disney0702
Oh you're welcome, I'm glad you found that useful.
May I ask what exam board you are and how old that question is?
May I ask what exam board you are and how old that question is?
I'm so sorry I thought that made a lot of sense but why are you reducing 120 ?
Original post by kandykissesxox
i thought it was a bent shape?
No clue if this picture is at all legible (Cameras a bit rubbish & my handwriting is scruffy).
The writing says 'two lone pair .'. 104.5' 'These two act as one lone pair' 'so trigonal planar and 120-2 = 118'
Can somebody help me with addition reactions and recations of alkenes i dont understand them. also if somebody could go over nucleophillic addition it would be greatly appreciated! 

Original post by kandykissesxox
It's AQA and the past paper was from 2002 

Ah okay I'm AQA too, and I'm now doing A2.
The reason why I asked as to how old it was was because the CCl2 shape is actually pretty different as we it has 2 bonding pairs and 1 lone pair and I don't think it is the specification to know the name of a shape for a molecule with that combination.
I thought it was best you know

I also attached a document that has all the Unit 1 topic by topic questions with the mark schemes, I used this last year to help me thought you might like it

Original post by Disney0702
Ah okay I'm AQA too, and I'm now doing A2.
The reason why I asked as to how old it was was because the CCl2 shape is actually pretty different as we it has 2 bonding pairs and 1 lone pair and I don't think it is the specification to know the name of a shape for a molecule with that combination.
I thought it was best you know
I also attached a document that has all the Unit 1 topic by topic questions with the mark schemes, I used this last year to help me thought you might like it
The reason why I asked as to how old it was was because the CCl2 shape is actually pretty different as we it has 2 bonding pairs and 1 lone pair and I don't think it is the specification to know the name of a shape for a molecule with that combination.
I thought it was best you know

I also attached a document that has all the Unit 1 topic by topic questions with the mark schemes, I used this last year to help me thought you might like it

Thank you so much!! This is really helpful xx
Original post by ThatPerson2
No clue if this picture is at all legible (Cameras a bit rubbish & my handwriting is scruffy).
The writing says 'two lone pair .'. 104.5' 'These two act as one lone pair' 'so trigonal planar and 120-2 = 118'
The writing says 'two lone pair .'. 104.5' 'These two act as one lone pair' 'so trigonal planar and 120-2 = 118'
Ah ok!! Thank you very much

Original post by kandykissesxox
I'm so sorry I thought that made a lot of sense but why are you reducing 120 ?
Ah right.
As you'll notice from the diagram in your question that they're trying to make students see a link between BCl3 and CCl2.
The link between these two is that both molecules have 3 bonds whether it be electron bonds or lone pairs.
The maximum bond angle for a molecule with 3 bonds is 120o, so this should be used as a base for students when tackling this question.
In CCl2 you'll see that one electron pair has been replaced by a lone pair and as we discussed earlier for every lone pair added we reduced the bond angle by 2.5o. This makes the bond angle in CCl2 117.5o.
Does that make more sense know?
Original post by Disney0702
Ah right.
As you'll notice from the diagram in your question that they're trying to make students see a link between BCl3 and CCl2.

The link between these two is that both molecules have 3 bonds whether it be electron bonds or lone pairs.
The maximum bond angle for a molecule with 3 bonds is 120o, so this should be used as a base for students when tackling this question.
In CCl2 you'll see that one electron pair has been replaced by a lone pair and as we discussed earlier for every lone pair added we reduced the bond angle by 2.5o. This makes the bond angle in CCl2 117.5o.
Does that make more sense know?
As you'll notice from the diagram in your question that they're trying to make students see a link between BCl3 and CCl2.
The link between these two is that both molecules have 3 bonds whether it be electron bonds or lone pairs.
The maximum bond angle for a molecule with 3 bonds is 120o, so this should be used as a base for students when tackling this question.
In CCl2 you'll see that one electron pair has been replaced by a lone pair and as we discussed earlier for every lone pair added we reduced the bond angle by 2.5o. This makes the bond angle in CCl2 117.5o.
Does that make more sense know?
Thank you very much! i understand
 It's a shame that you have to memorise that whole table
It's a shame that you have to memorise that whole table 
Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- Chemistry Degree Lab Work
- Making chemistry notes
- Physical natural sciences
- !!! A level choices - Chem or Computer science
- A level chemistry for a biology degree? (help please!!)
- Do I have enough time to turn my grades around??
- Mass vs relative atomic mass on periodic table
- Official NATURAL SCIENCES applicants thread 2024
- What is it like doing chem vs natural sciences at uni?
- Study leave
- in need of urgent advice, would appreciate any help :)
- Lancaster uni timetable
- Need help on a Venn diagram question
- Biochemistry at University
- Cambridge Natural Sciences Interview Applicants 2023/24
- Higher chemistry
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me?
- Save me from a level torture
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



