AS-Level Chemistry help?!
Any chemistry revision tips? And anything that makes the maths in it any easier! 

Original post by XcitingStuart
These are tips for biology and chemistry.
Biology
Make notes, learn, do questions, use questions to rewrite notes so you're learning the correct stuff. (P.S. Do all the questions you can, to include anything possibly relevant, better the earlier, so you're learning the correct stuff the earliest. With application of knowledge, you don't need to necessarily learn it, unless you think you definitely won't all of get it in the exam.)
After you've learnt something,make sure to revisit it; I mean it. I'll tell you this story from experience, I got an A at BIO1 past paper in January, so I presumed I'd remember it, so I revisited it really late. I never revisited it earlier, because I never realised I forgot so much stuff. Luckily short-term memory can be a blessing.
Do past paper questions for biology; honestly, they cover everything the best.
So write your final notes off the past papers, golden rule.
Make sure you also confer with your teachers about what you need to know.
Chemistry
Luckily most things in chemistry complement each other.
Some things, you'll learn, just take a while to fully comprehend.
I'd say chemistry is far easier than biology in terms of workload.
Ionisation energies, they require some time to fully comprehend, like when you learn later about a thing called electronegativities (don't be daunted, very easy) you solidify your understanding of ionisation energies more. You get a better understanding for how the size of the atom relates, for example.
With mass spectrometry, I'd say to just see a mark scheme answer on it, as although it's long, it's very logical, but this includes all the info needed. (I might upload my own notes for you.)
Chemical calculations can be summarised up into about 5 calculations, very basic algebra is needed here.
Do you know how to use triangles for equations with 3 variables?
So imagine the equation n = m/M

Now, this triangle can work for so many equations (all equations with only 3 variables.)
To work out n, simply cover n then look at it; it's m/M,
for m, it's n x M,
for M, it's m/n.
Only one thing can ever go at the top, so if you know the equation (any with 3 variables), you can instantly configure the triangle.
Sorry if this stuff seems very basic to you, but many people can have trouble with it.
Onto another equation, n=c x v, where would these go?
.Hess's law, now this confused me greatly at first (perhaps I wasn't paying enough attention, I don't know). Once you get it fully, you get it, but getting there... I just didn't get it when a teacher was explaining it to me (though I don't think it was the teacher's fault; sometimes it just happens.) I saw other things online explaining it to me, but when I saw a specific one, it was like the person who made it thought exactly like me, and I got it instantly.
Need to do some questions to make sure you get into the habit of not doing silly mistakes, if that makes any sense.
I'll upload my own notes for this, probably. My outline for this was amazing.
Hydrogen spectrum, easy, logical theory (nice because chemistry is all a, then b, then c, well it feels like it anyway.) Mark scheme should be fine here.
CH2
Organic chemistry, mechanisms, it's like it's easier to fully memorise the drawn equation before you can fully understand it in your own head, also without looking at your notes. I found I only fully understood it, after I fully memorised the equation(s).
If you know the rules about electronegativities (technically, the electrons will go to the more electronegative elements, the more electronegative element is the element closer to fluorine), it's very logical, and very easy later on.
So if a carbon - oxygen bond was going to split, where would the two electrons (constituting the covalent bond) go to? which element? (If the most electronegative is closer to fluorine.)Periodicities (periodic trends) just memorise, gets easier later on (there is logic though.) Loads of nick-knacks here though, unfortunately.
When it goes onto the shapes of molecules, fortunately for me, I was privvy to some really good resources, which you would probably be unable to get to, considering my college paid a lot to get to them. :/ Quote me when you get to this part (towards the end of the year) and I give you some immensely helpful tips. It'd kinda be too wordy at the moment, if you have no idea what I'm talking about. (It'd be too wordy for this post, already long, regardless.)
Some other minor topics you should be fine with.
With chemistry, on a last note, you can literally just work with past papers to do practically all your revision (especially in CH1.)(In CH2, you'll still need to memorise specific parts, but it ain't that bad. You quickly get what is wanted for you.)
Biology will take longer than chemistry; far more to memorise. Key here is mark scheme, to remember the right things. Although this is said a lot, seriously, it's just better to start working earlier, to put less stress on you later (and I mean a lot less stress.) I'm not saying it's impossible to get good grades if you don't start it early, it'd just make you one unhappy bunny.
I'd say again, if you need help with anything specific, message/reply to me; I can help, having done an AS in both, and know the tricks or easy ways to remember things.
Biology
Make notes, learn, do questions, use questions to rewrite notes so you're learning the correct stuff. (P.S. Do all the questions you can, to include anything possibly relevant, better the earlier, so you're learning the correct stuff the earliest. With application of knowledge, you don't need to necessarily learn it, unless you think you definitely won't all of get it in the exam.)
After you've learnt something,make sure to revisit it; I mean it. I'll tell you this story from experience, I got an A at BIO1 past paper in January, so I presumed I'd remember it, so I revisited it really late. I never revisited it earlier, because I never realised I forgot so much stuff. Luckily short-term memory can be a blessing.
Do past paper questions for biology; honestly, they cover everything the best.
So write your final notes off the past papers, golden rule.
Make sure you also confer with your teachers about what you need to know.
Chemistry
Luckily most things in chemistry complement each other.
Some things, you'll learn, just take a while to fully comprehend.
I'd say chemistry is far easier than biology in terms of workload.
Ionisation energies, they require some time to fully comprehend, like when you learn later about a thing called electronegativities (don't be daunted, very easy) you solidify your understanding of ionisation energies more. You get a better understanding for how the size of the atom relates, for example.
With mass spectrometry, I'd say to just see a mark scheme answer on it, as although it's long, it's very logical, but this includes all the info needed. (I might upload my own notes for you.)
Chemical calculations can be summarised up into about 5 calculations, very basic algebra is needed here.
Do you know how to use triangles for equations with 3 variables?
So imagine the equation n = m/M
Now, this triangle can work for so many equations (all equations with only 3 variables.)
To work out n, simply cover n then look at it; it's m/M,
for m, it's n x M,
for M, it's m/n.
Only one thing can ever go at the top, so if you know the equation (any with 3 variables), you can instantly configure the triangle.
Sorry if this stuff seems very basic to you, but many people can have trouble with it.
Onto another equation, n=c x v, where would these go?
.
Spoiler
Need to do some questions to make sure you get into the habit of not doing silly mistakes, if that makes any sense.
I'll upload my own notes for this, probably. My outline for this was amazing.
Hydrogen spectrum, easy, logical theory (nice because chemistry is all a, then b, then c, well it feels like it anyway.) Mark scheme should be fine here.
CH2
Organic chemistry, mechanisms, it's like it's easier to fully memorise the drawn equation before you can fully understand it in your own head, also without looking at your notes. I found I only fully understood it, after I fully memorised the equation(s).
If you know the rules about electronegativities (technically, the electrons will go to the more electronegative elements, the more electronegative element is the element closer to fluorine), it's very logical, and very easy later on.
So if a carbon - oxygen bond was going to split, where would the two electrons (constituting the covalent bond) go to? which element? (If the most electronegative is closer to fluorine.)
Spoiler
When it goes onto the shapes of molecules, fortunately for me, I was privvy to some really good resources, which you would probably be unable to get to, considering my college paid a lot to get to them. :/ Quote me when you get to this part (towards the end of the year) and I give you some immensely helpful tips. It'd kinda be too wordy at the moment, if you have no idea what I'm talking about. (It'd be too wordy for this post, already long, regardless.)
Some other minor topics you should be fine with.
With chemistry, on a last note, you can literally just work with past papers to do practically all your revision (especially in CH1.)(In CH2, you'll still need to memorise specific parts, but it ain't that bad. You quickly get what is wanted for you.)
Biology will take longer than chemistry; far more to memorise. Key here is mark scheme, to remember the right things. Although this is said a lot, seriously, it's just better to start working earlier, to put less stress on you later (and I mean a lot less stress.) I'm not saying it's impossible to get good grades if you don't start it early, it'd just make you one unhappy bunny.
I'd say again, if you need help with anything specific, message/reply to me; I can help, having done an AS in both, and know the tricks or easy ways to remember things.
Original post by XcitingStuart
About the triangles for equations, you should do this in your head later, just imagine the triangle with the variables input.
Just go to the relevant parts.
Original post by XcitingStuart
Just go to the relevant parts.
Omg you wrote all that? I never got hess' law myself
 do you remember what you watched?
do you remember what you watched?Original post by XcitingStuart
Just go to the relevant parts.
Wow! Thanks a lot, that's sure to help!

Original post by TheonlyMrsHolmes
Omg you wrote all that? I never got hess' law myself  do you remember what you watched?
do you remember what you watched?
 do you remember what you watched?
do you remember what you watched?Here are my notes for Hess's law equation.
it has accounted for everyone up until you might need to rearrange the ΔH = ΔH1 + ΔH2
(let's say if you got the values ΔH and ΔH1, to rearrange it to get ΔH2, you'd need to do ΔH2 = ΔH - ΔH1, I think.)
It has never failed me, so if you get the wrong answer, you're misinterpreting my notes.
One other thing it has forgot to mention, is if it is bond enthalpies, the reaction starts at the elements, so the arrows are going away. (edit #1: silly me, I made a mistake here. Ignore bond enthalpy arrow direction until I confirm something; I haven't explained this the best.)
I actually invested a lot of time into my notes (just before January mock I spent the week(s) before catching up, and it nearly killed me metaphorically speaking. I was still clueless about how to revise. And the exhaustion and stress I put myself through was, ouch.) So I don't mind you quoting them elsewhere, but please can you give credit where it's due?
After some practice you'll learn how to do them in like 15 seconds in your head, honestly.
Attachment not found
@skylightflower
@Jkoo22
@AnimeFreak101
@Ser Alex Toyne
@hopefulmedic1998
@jackreid
@SampleX
(edited 8 years ago)
Edit #1: Ignore for now, there's something wrong with this.
Edit #2: Fixed.
I'll give an example
This is a tiny bit of A2
-[3(348)+3(612)+6(412)]ΔHf = 246 kJmol-1
See how the first arrow's going up,
if you go around the first arrow against the gradient, so you minus it
the second arrow is with, so you plus it.
It's hard to explain without being there in person.
Edit #2: Fixed.
I'll give an example
This is a tiny bit of A2
Spoiler
6C + 3H2(g) → C6C6
↓------------------------↓
C + H
+[6(715) + 3(436)]↓------------------------↓
C + H
-[3(348)+3(612)+6(412)]ΔHf = 246 kJmol-1
See how the first arrow's going up,
if you go around the first arrow against the gradient, so you minus it
the second arrow is with, so you plus it.
It's hard to explain without being there in person.

(edited 8 years ago)
I'll re-explain my notes above, because they're kind of confusing .
PART 1
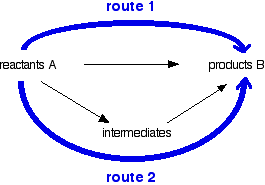
Hess's Law states that the enthalpy change of a reaction is independent of the route taken.
This technically:

ΔH, the black arrow on top.
ΔH1, the black arrow to the left.
ΔH2, the black arrow to the right.
ΔH = ΔH1 + ΔH2
See the relationship?
Understand this before you move on.
PART 1
Hess's Law states that the enthalpy change of a reaction is independent of the route taken.
This technically:

ΔH, the black arrow on top.
ΔH1, the black arrow to the left.
ΔH2, the black arrow to the right.
ΔH = ΔH1 + ΔH2
See the relationship?
Understand this before you move on.
PART 2
Your reaction of interest goes at the top.
The direction of your arrows depends on whether you're doing
a) enthalpies of formation
The standard enthalpy change of formation means "the enthalpy change when one mole of a compound in its standard state is formed from its elements in its standard state, under standard conditions".
If formation enthalpies are used, the reaction is starting with elements, so the elements have the arrows pointing away.
b) enthalpies of combustion
The standard enthalpy change of combustion means "the enthalpy change when one mole of an element or compound is completely burned in excess oxygen under standard conditions".
If combustion enthalpies are used, the reaction ends with combustion products, so the combustion products have arrows pointing towards them.
c) bond enthalpies
The standard bond enthalpy change means "the enthalpy change when one mole of a particular chemical bond is broken in the gaseous state to give gaseous atoms under standard conditions".
If bond enthalpies are used, the reaction starts with the elements, so the elements have arrows pointing away from them.
See in the picture below...

Those two arrows in blue are always going to be kept the same (in this method.)
You must though, change the black arrows in-between.
Now, if black arrow (ΔH1) from e.g. reactants A to the intermediates is in alignment to the route two blue arrow, you plus the ΔH1. If it goes against the route two blue arrow, you minus ΔH1.
Exactly the same with ΔH2. If in alignment, you plus, if in opposition, you minus.
Now you arrive to inputting the values into ΔH = ΔH1 + ΔH2.
usually they'll just ask you to find ΔH, but they occasionally ask you to find ΔH2, or ΔH1, then you have you just rearrange the equation at the end.
So
ΔH1 = ΔH - ΔH2
ΔH2 = ΔH1 + ΔH1
Done! Please say if you don't understand, it's far harder to explain via the Internet.
Your reaction of interest goes at the top.
The direction of your arrows depends on whether you're doing
a) enthalpies of formation
The standard enthalpy change of formation means "the enthalpy change when one mole of a compound in its standard state is formed from its elements in its standard state, under standard conditions".
If formation enthalpies are used, the reaction is starting with elements, so the elements have the arrows pointing away.
b) enthalpies of combustion
The standard enthalpy change of combustion means "the enthalpy change when one mole of an element or compound is completely burned in excess oxygen under standard conditions".
If combustion enthalpies are used, the reaction ends with combustion products, so the combustion products have arrows pointing towards them.
c) bond enthalpies
The standard bond enthalpy change means "the enthalpy change when one mole of a particular chemical bond is broken in the gaseous state to give gaseous atoms under standard conditions".
If bond enthalpies are used, the reaction starts with the elements, so the elements have arrows pointing away from them.
See in the picture below...

Those two arrows in blue are always going to be kept the same (in this method.)
You must though, change the black arrows in-between.
Now, if black arrow (ΔH1) from e.g. reactants A to the intermediates is in alignment to the route two blue arrow, you plus the ΔH1. If it goes against the route two blue arrow, you minus ΔH1.
Exactly the same with ΔH2. If in alignment, you plus, if in opposition, you minus.
Now you arrive to inputting the values into ΔH = ΔH1 + ΔH2.
usually they'll just ask you to find ΔH, but they occasionally ask you to find ΔH2, or ΔH1, then you have you just rearrange the equation at the end.
So
ΔH1 = ΔH - ΔH2
ΔH2 = ΔH1 + ΔH1
Done! Please say if you don't understand, it's far harder to explain via the Internet.
(edited 8 years ago)
You have to have some sort of gist to even have a clue as to what I'm talking about.
(That's why I included the definitions, to clarify.)
(That's why I included the definitions, to clarify.)
Sorry, I forgot to quote you again for the updated version.
Updated version has a lot more clarity.
@TheonlyMrsHolmes
@skylightflower
@Jkoo22
@AnimeFreak101
@Ser Alex Toyne
@hopefulmedic1998
@jackreid
@SampleX
Updated version has a lot more clarity.
@TheonlyMrsHolmes
@skylightflower
@Jkoo22
@AnimeFreak101
@Ser Alex Toyne
@hopefulmedic1998
@jackreid
@SampleX
Original post by XcitingStuart
Sorry, I forgot to quote you again for the updated version.
Updated version has a lot more clarity.
Updated version has a lot more clarity.
Thank you for that! I will have a look through it, it's very detailed so thank you!
Quick Reply
Related discussions
- chemistry or psychology a level??
- TSR Study Together - STEM vs Humanities!
- A level chemistry for a biology degree? (help please!!)
- A Level Choices Help (Aqa Physics V Chemistry)
- pls be my chemistry tutor !!
- Chemistry A-level
- Chemistry or physics A level
- What university has the lowest GCSE requirements for medicine?
- I'm unsure whether I should do chemistry A levels
- Is it worth doing chemistry for a neuroscience degree?
- a level chemistry
- Is there anyway to do extra chemistry outside of school?
- A-level combo for Pharmacy
- Worried about degree difficulty - Chemistry
- Can I go into biochem or work in the medical field with these alevels?
- How to make my application strong
- Biomed vs Biochem vs chemistry
- Oxford Chemistry?
- Biomed / Pharmacy
- Chemistry A level
Latest
Last reply 1 minute ago
NICS Staff Officer and Deputy Principal recruitment 2022 2023Last reply 2 minutes ago
Rishi Sunak pledges to remove benefits for people not taking jobs after 12 monthsLast reply 3 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 5 minutes ago
Can I do medicine with these gcse grades and what unis would be most likely to acceptPosted 7 minutes ago
Worried about son who wants to move out, will he be able to afford it?Last reply 8 minutes ago
Work experience opportunity related to finance/economiczLast reply 12 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 12 minutes ago
year 13 gyg journal : trying not to become an academic victim 🤡📖Last reply 13 minutes ago
What do you like most about having a Grow your Grades blog?Last reply 15 minutes ago
Northeastern University London Essay Competition



