Aqa chem 4/ chem 5 june 2016 thread
Scroll to see replies
Original post by ahsan_ijaz
Hi,
Well since your adding extra oh- ions they react with the H+ when acid dissociate
So HX= H+ + X-
When you add extra OH- ions the OH- will react with the H+ to form water thus the H+ conc is reduced at the time. However, We know the H+ conc has been reduced so the ph should drop but since we know a buffer solution is one that resists changes in Ph when small ammount of alkali/acid is added. The equilibrium will shift to the right to replace the lost H+ which will bring the pH back close to its original value
hope it helps
Well since your adding extra oh- ions they react with the H+ when acid dissociate
So HX= H+ + X-
When you add extra OH- ions the OH- will react with the H+ to form water thus the H+ conc is reduced at the time. However, We know the H+ conc has been reduced so the ph should drop but since we know a buffer solution is one that resists changes in Ph when small ammount of alkali/acid is added. The equilibrium will shift to the right to replace the lost H+ which will bring the pH back close to its original value
hope it helps
Nice explanation!
I'm always tempted to write that the oh- reacts with HX to form X-, but because the [HX] and [X-] are so high relative to the [oh-], that the ratio of the [HX]/[X-] effectively remains constant, so the solution resists a change in pH despite a small amount of alkali being added... do you think this would be accepted too?
It's rarely on, not many mark schemes to see
Original post by DanMargetts
Nice explanation!
I'm always tempted to write that the oh- reacts with HX to form X-, but because the [HX] and [X-] are so high relative to the [oh-], that the ratio of the [HX]/[X-] effectively remains constant, so the solution resists a change in pH despite a small amount of alkali being added... do you think this would be accepted too?
It's rarely on, not many mark schemes to see
I'm always tempted to write that the oh- reacts with HX to form X-, but because the [HX] and [X-] are so high relative to the [oh-], that the ratio of the [HX]/[X-] effectively remains constant, so the solution resists a change in pH despite a small amount of alkali being added... do you think this would be accepted too?
It's rarely on, not many mark schemes to see
Hi,
M1 extra/added OH–removed by reaction with H+or the acid
M2 correct discussion of equm shift i.e. HXH++ X– movesto right
ORratio[X ][HX]-remains almost constant
This is legit the marks scheme i found regarding this Q so its what the examiner expects from us - However , I think what you have said is on this mark scheme and their is no reason to believe that it wont be on it in the future so it should be accepted as it makes sense in terms of chemistry- Rememebr the examiners most of them are qualified teachers etc so if they think that your talking sense you shall be definitely awarded for it as i dont see anything in particular underlined on this ms answer.
Can someone explain 3e and 3eii to me.
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Also 3a, Why cant you have c3h7?
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Also 3a, Why cant you have c3h7?
Original post by ahsan_ijaz
Hi,
M1 extra/added OH–removed by reaction with H+or the acid
M2 correct discussion of equm shift i.e. HXH++ X– movesto right
ORratio[X ][HX]-remains almost constant
This is legit the marks scheme i found regarding this Q so its what the examiner expects from us - However , I think what you have said is on this mark scheme and their is no reason to believe that it wont be on it in the future so it should be accepted as it makes sense in terms of chemistry- Rememebr the examiners most of them are qualified teachers etc so if they think that your talking sense you shall be definitely awarded for it as i dont see anything in particular underlined on this ms answer.
M1 extra/added OH–removed by reaction with H+or the acid
M2 correct discussion of equm shift i.e. HXH++ X– movesto right
ORratio[X ][HX]-remains almost constant
This is legit the marks scheme i found regarding this Q so its what the examiner expects from us - However , I think what you have said is on this mark scheme and their is no reason to believe that it wont be on it in the future so it should be accepted as it makes sense in terms of chemistry- Rememebr the examiners most of them are qualified teachers etc so if they think that your talking sense you shall be definitely awarded for it as i dont see anything in particular underlined on this ms answer.
Thank you for the advice, I appreciate the hopeful words! All the best with the exams

Original post by string210
thats what i did, checked the mark scheme, it wasnt right!
If you work out the moles of both the acid and the base you'll find that the moles of OH- = moles of H+. This is the half equivalence point.
Here's my working if you're interested. It's a nice property to remember which will save you some time when working but the procedure is exactly the same for any neutralization reaction.

Original post by DanMargetts
Nice explanation!
I'm always tempted to write that the oh- reacts with HX to form X-, but because the [HX] and [X-] are so high relative to the [oh-], that the ratio of the [HX]/[X-] effectively remains constant, so the solution resists a change in pH despite a small amount of alkali being added... do you think this would be accepted too?
It's rarely on, not many mark schemes to see
I'm always tempted to write that the oh- reacts with HX to form X-, but because the [HX] and [X-] are so high relative to the [oh-], that the ratio of the [HX]/[X-] effectively remains constant, so the solution resists a change in pH despite a small amount of alkali being added... do you think this would be accepted too?
It's rarely on, not many mark schemes to see
hey wat do you mean when you say that the concentration of HX and X- are so high?
Original post by Lilly1234567890
hey wat do you mean when you say that the concentration of HX and X- are so high?
Relative to the amount of H+ added
Or am I wrong? It could be that their moles are a lot higher, in buffer questions, the mol of H+ added is always really small, but often the concentration is too, so I guess there the mol and concentration of HX and X- are higher than the mol and conc of H+, if that makes sense..
Original post by Super199
Can someone explain 3e and 3eii to me.
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Also 3a, Why cant you have c3h7?
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Also 3a, Why cant you have c3h7?
3e) a primary amine and the carbon skeleton has 3 different environments for carbon so
H2N - CH2 - C (CH3)3
This has three different carbon environments and is a primary amine.
3ei) this has 3 hydrogen environments (the two methyl groups are in the same environment) and the H on the carbon causes doublet splitting on the adjacent methyl groups
(CH3)2-C-H
I
CH3 - N - CH3
Original post by emma_1111
For acid base questions if the acid is in XS do you divide the XS by the total volume or do you only do that if the OH is in XS?
always divide by volume
Original post by Parallex
If you work out the moles of both the acid and the base you'll find that the moles of OH- = moles of H+. This is the half equivalence point.
Here's my working if you're interested. It's a nice property to remember which will save you some time when working but the procedure is exactly the same for any neutralization reaction.

Here's my working if you're interested. It's a nice property to remember which will save you some time when working but the procedure is exactly the same for any neutralization reaction.

Thank you so much for this! Need to look out for this on Tuesday
Original post by lahigueraxxx
Thank you so much for this! Need to look out for this on Tuesday
There's no need to remember specifics like the half equivalence point, that's just a little niche thing that can save you a bit of time. Just know that a neutralisation reaction will occur if OH- is added so you need to work out the new moles.
Could somebody explain to me why the answer is P on June 2015 Q10c)? At first I thought that it could be S or T - don't S and T also have 5 peaks? The number of peaks for S and T must be greater than 5 but I don't understand why.
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN15.PDF
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN15.PDF
4b
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Can someone explain how to work out the number of molecular ion peaks?
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Can someone explain how to work out the number of molecular ion peaks?
Original post by Aerosmith
Could somebody explain to me why the answer is P on June 2015 Q10c)? At first I thought that it could be S or T - don't S and T also have 5 peaks? The number of peaks for S and T must be greater than 5 but I don't understand why.
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN15.PDF
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN15.PDF
S and T both have 6 peaks. For S, there are 5 non-equivalent H peaks in the ring structure and 1 on the OH. For T, the same applies for the ring and the methyl group.
Original post by Super199
4b
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Can someone explain how to work out the number of molecular ion peaks?
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-W-QP-JUN10.PDF
Can someone explain how to work out the number of molecular ion peaks?
There are 4 chlorine atoms in the compound which exist as Cl-35 and Cl-37. There's 5 different combinations of the isotopes:
1) Cl-35 - Cl-35 - Cl-35 - Cl-35
2) Cl-37 - Cl-37 - Cl-37 - Cl-37
3) Cl-35 - Cl-35 - Cl-35 - Cl-37
4) Cl-35 - Cl-35 - Cl-37 - Cl-37
5) Cl-35 - Cl-37 - Cl-37 - Cl-37
Original post by Parallex
There are 4 chlorine atoms in the compound which exist as Cl-35 and Cl-37. There's 5 different combinations of the isotopes:
1) Cl-35 - Cl-35 - Cl-35 - Cl-35
2) Cl-37 - Cl-37 - Cl-37 - Cl-37
3) Cl-35 - Cl-35 - Cl-35 - Cl-37
4) Cl-35 - Cl-35 - Cl-37 - Cl-37
5) Cl-35 - Cl-37 - Cl-37 - Cl-37
1) Cl-35 - Cl-35 - Cl-35 - Cl-35
2) Cl-37 - Cl-37 - Cl-37 - Cl-37
3) Cl-35 - Cl-35 - Cl-35 - Cl-37
4) Cl-35 - Cl-35 - Cl-37 - Cl-37
5) Cl-35 - Cl-37 - Cl-37 - Cl-37
I see, how would you work out the number of carbon peak 4dii
Original post by Super199
I see, how would you work out the number of carbon peak 4dii
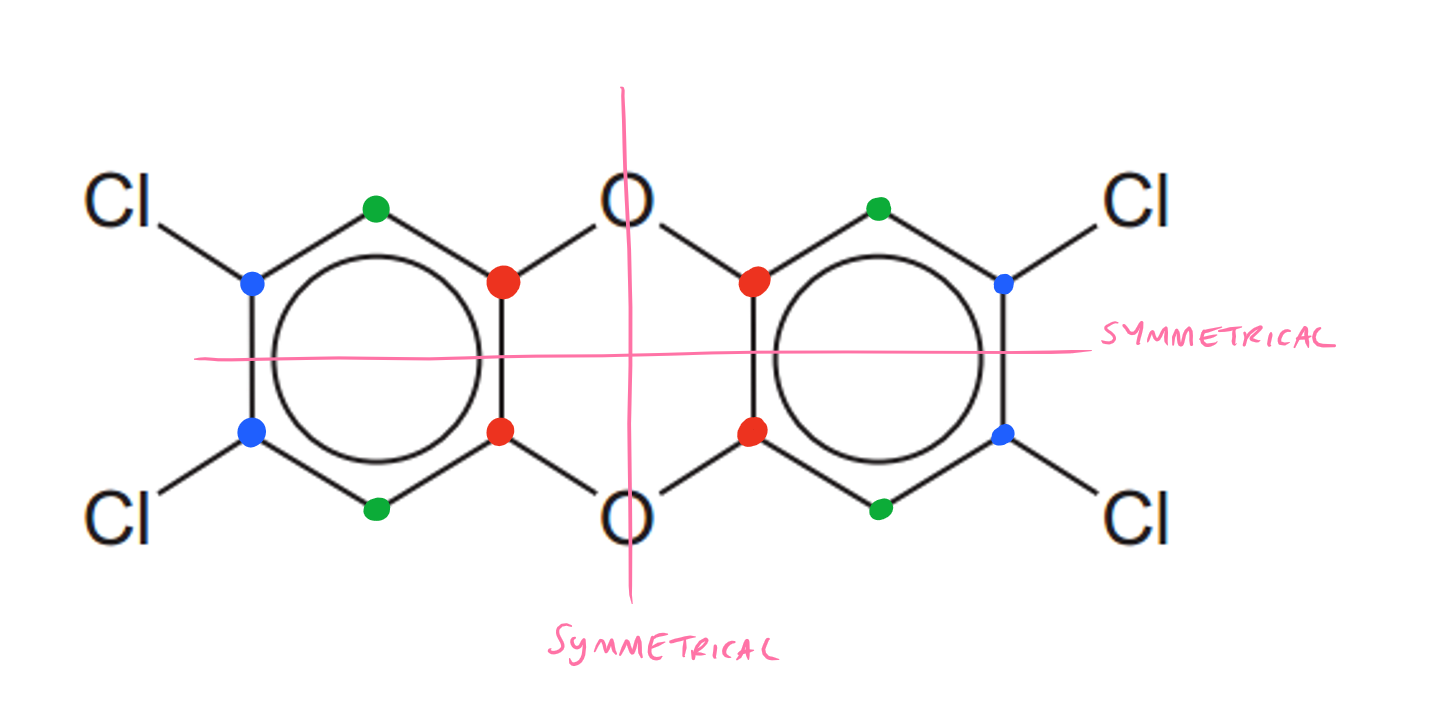
Equivalent carbon groups are the same colour. The compound is symmetrical about the O bonds so you only need to consider one half of the structure. Splitting the compound horizontally there is also a symmetry, so the groups below are equivalent to the groups above. There are 3 non-equivalent carbon groups in the molecule.
Quick Reply
Related discussions
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- GCSE Exam Discussions 2024
- TSR Study Together - STEM vs Humanities!
- A-level Exam Discussions 2024
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- reuben's y13/medapps journey!!
- A-level Biology Study Group 2022-2023
- Need Jan 2022 Past papers - Oxford AQA international A level BL05
- Grade Growth Chronicles | From C's to A's (23-24)
- Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05
- better late than never - gyg 24' :)
- GCSE Results: Post your results
- Holding myself accountable; study!
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
- A Level Exam Discussions 2023
- C in Biology and D in Chem, while I resit could I do an intensive alevel in maths
- GYG a level y13⋆୨୧˚⟡˖ ࣪
- AS chemistry paper 2 2022 AQ
- AQA A Level Chemistry Paper 1 Practicals
- Biology Paper 1 - PRACTICE exam paper (with mark scheme) INVALUABLE resource
Latest
Last reply 1 minute ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 1 minute ago
anyone else waiting on LSE law?Last reply 2 minutes ago
Software Engineering Degree Apprenticeship | Digital & Technology Solutions Level 6Last reply 2 minutes ago
Civil service - location allocations and preference DWPLast reply 2 minutes ago
Government Social Research - Research Officer Scheme 2024Last reply 3 minutes ago
PA Consulting Degree Apprenticeships 2024Last reply 3 minutes ago
Imperial MSc Machine Learning and Data Science (Online via Coursera)Last reply 5 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 7 minutes ago
Official University of St Andrews Applicant Thread for 2024Last reply 7 minutes ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadLast reply 8 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 9 minutes ago
yet to receive a woodhouse interviewLast reply 10 minutes ago
Michaela School: Muslim student loses prayer ban challengeLast reply 15 minutes ago
Official: Imperial College London A100 2024 Entry ApplicantsTrending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



