OCR Chemistry - Why does H2S have a lower boiling point than H2Se?
I understand that H2O has a higher boiling point than H2S because it forms Hydrogen Bonds between its molecules which are strong.
BUT why does H2S have a lower boiling point than H2Se?
I thought they were both polar molecules in which case surely they form permanent dipole forces?
BUT why does H2S have a lower boiling point than H2Se?
I thought they were both polar molecules in which case surely they form permanent dipole forces?
Original post by glastonbury
I understand that H2O has a higher boiling point than H2S because it forms Hydrogen Bonds between its molecules which are strong.
BUT why does H2S have a lower boiling point than H2Se?
I thought they were both polar molecules in which case surely they form permanent dipole forces?
BUT why does H2S have a lower boiling point than H2Se?
I thought they were both polar molecules in which case surely they form permanent dipole forces?
Go to the periodic table ,look at Se and S,see which one has more electrons and then think about London forces.
Okay, but why induced dipole (london) forces? Surely if they are polar molecules they form permanent dipole forces?
Original post by glastonbury
Okay, but why induced dipole (london) forces? Surely if they are polar molecules they form permanent dipole forces?
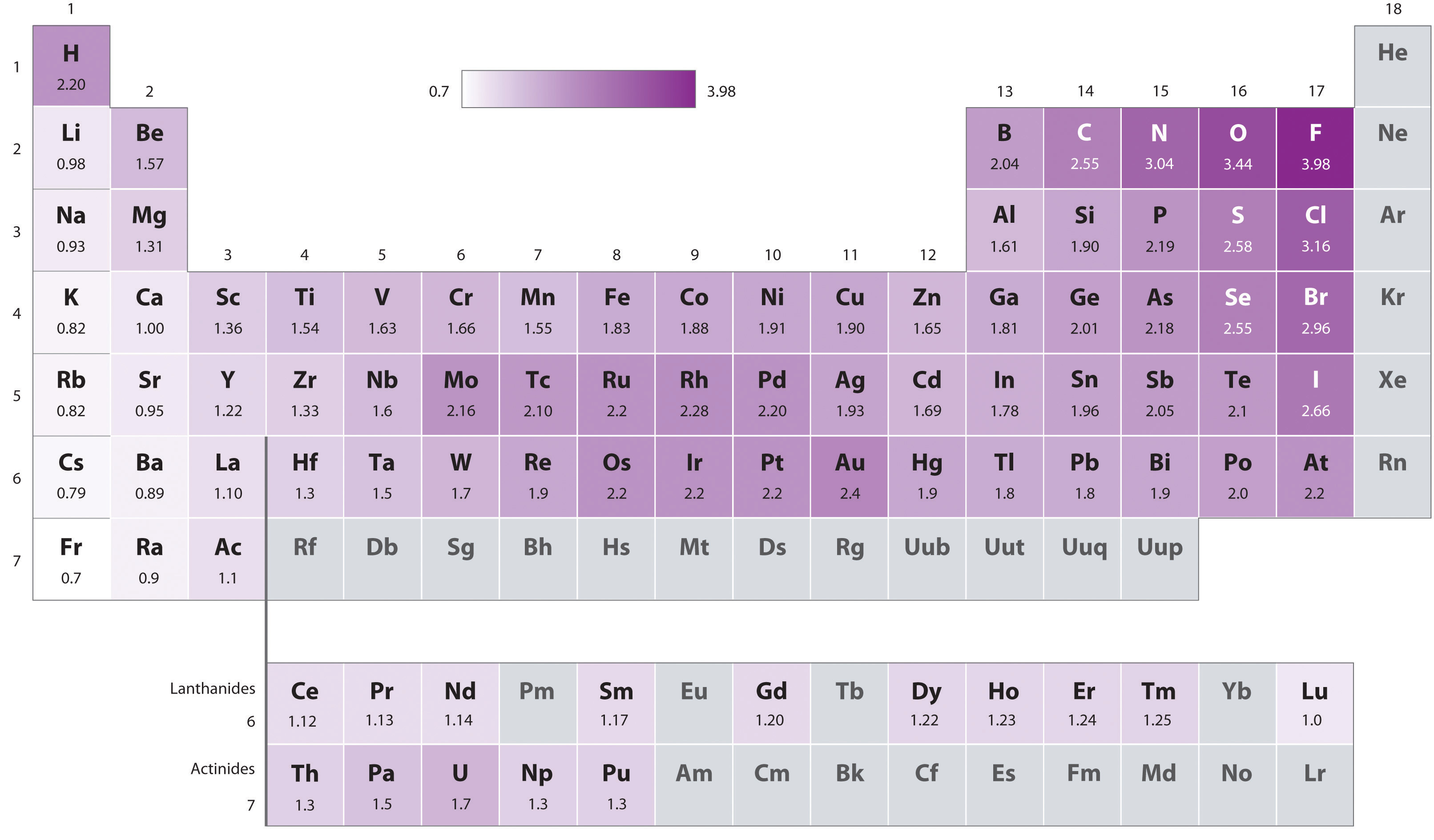
The electronegativity difference is too small.
Original post by glastonbury
Okay, but why induced dipole (london) forces? Surely if they are polar molecules they form permanent dipole forces?

The electronegativity difference is too small.
Original post by glastonbury
Okay, but why induced dipole (london) forces? Surely if they are polar molecules they form permanent dipole forces?
It is very important to know that permanent dipoles occur in addition to London forces not instead! Also the bigger the atom the higher the London forces.
Originally, I thought h2se would have a lower boiling point since it has more shielding and a larger atomicis radius meaning that the attraction between the nucleus and the outer electrons is weaker and there being easier to break. I also thought that the atomic number ( amount of protons & electrons) was overruled by shielding so it wouldn’t matter if h2se has more electrons than h2s cox it has more shielding and therefore should be lower BP. But in this question it says it’s higher h2se has a higher BP than h2s so tbh I think all you have to mention is that h2se has more electrons and protons so it has a stronger attraction from nucleus and outer electrons and I think we completely ignore shielding which I don’t really get why??? I find it weird that h2se is more electronegative considering that electro negativity increase up and along the periodic table and h2s it up higher in periodic table than h2se
The reason shielding, nuclear attraction and atomic radius don’t have an impact on the boiling point is because this question is talking about intermolecular forces (the forces holding molecules together, not atoms - that’s bonding). In order to change states, you have to break the forces holding molecules together - if you remember solids have molecules that are very tightly packed whereas gases have molecules that’s are dispersed. H2Se has stronger London forces than H2S because it has more electrons meaning the induced dipoles will be stronger - more energy is needed to break them so the boiling point is higher. Hopefully that makes sense

Original post by glastonbury
I understand that H2O has a higher boiling point than H2S because it forms Hydrogen Bonds between its molecules which are strong.
BUT why does H2S have a lower boiling point than H2Se?
I thought they were both polar molecules in which case surely they form permanent dipole forces?
BUT why does H2S have a lower boiling point than H2Se?
I thought they were both polar molecules in which case surely they form permanent dipole forces?
If you look at the periodic table Selenium is one block below sulfur and is hence a bigger atom as it has got one extra electron shell. This essentially means H2Se is a bigger molecule than H2S meaning the Van Der Waal forces (London forces) on H2Se is stronger than on H2S which means it takes more energy to break the intermolecular forces for H2Se than H2S- this leads to H2S having the lower boiling point.
However a conflicting idea is that sulfur is more electronegative than selenium so you may think the permanent dipole dipole forces will be stronger for H2S and you are right. But its just the fact that the stronger Van Der Waal forces is the overriding factor in what determines the overall boiling point in this case.
Quick Reply
Related discussions
- Chemistry - melting point
- why reflux? (alcohol to ketone)
- A-level Chemistry Study Group 2022-2023
- A level chemistry
- Boiling point of halogenoalkanes
- Interpreting UCAS offer
- if something has a high boiling point does it have a high melting point a level?
- OCR A GCSE Chemistry Paper 3 Higher Tier (J248 03) - 22nd May 2023 [Exam Chat]
- Help polymers
- Singapore applicant to UK help
- OCR A-Level Chemistry B Paper 1 (H433/01) - 12th June 2023 [Exam Chat]
- AS/A Level Chemistry Study Group 2023/2024
- OCR A Level Chemistry Paper 1 H432/01 - 13th Jun 2022 [Exam Chat]
- AQA GCSE Chemistry Paper 1 Higher Tier (8462 1H) - 22nd May 2023 [Exam Chat]
- A level revision songs
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- Further Maths Modules
- chemistry
- AS Chemistry Multiple choice question help TQ
- AQA A-Level Chemistry Paper 3 (7405/3) - 23rd June 2023 [Exam Chat]
Latest
Last reply 5 minutes ago
National Probation ServiceLast reply 7 minutes ago
Home Office: Immigration Enforcement Casework Support AO 2024Last reply 8 minutes ago
Astrazeneca/pharmaceutical degree apprenticeships 2024Last reply 11 minutes ago
If the Russell Group was now a fair representation of what it still claims to beLast reply 14 minutes ago
ATAS (Academic, Technology, Approval Scheme) Certificate 2023/2024Last reply 15 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2908
Last reply 15 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 16 minutes ago
Which of these A-Level combinations would be the best to study a law degree?A-levels
10
Last reply 17 minutes ago
Border Force National Campaign September 2023Last reply 21 minutes ago
lloyds bank 2024 apprenticeshipLast reply 21 minutes ago
Weidenfeld Hoffmann Trust (WHT) Scholarship Notification (2024-2025)Last reply 27 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 29 minutes ago
Can a law graduate at SOAS apply to MSc Politics and International Relations at soas?Posted 32 minutes ago
SQA Higher Computing Studies - 20th May 2024 [Exam Chat]Trending
Last reply 2 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]Trending
Last reply 2 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]



