Chemical structure of oxytocin doesn't make sense?
Look at the Glycine at the beginning, why is the CO connected to an NH2 when it's supposed to be a carboxylic acid? Makes no sense. The structure of glycine is NH2-CH2-COOH not NH2-CO-CH2-NH2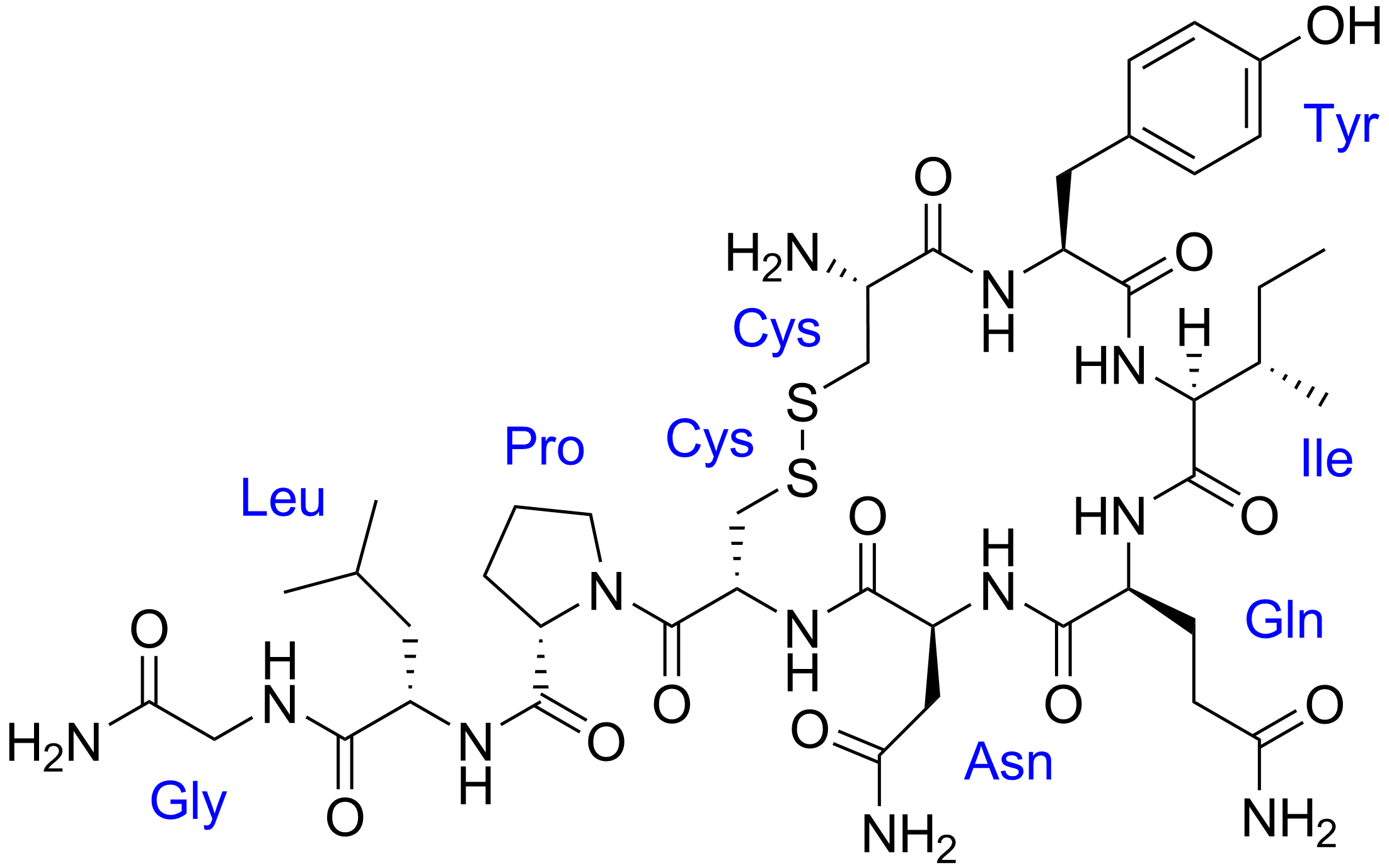

Original post by alow
What do you mean it's "supposed" to be a carboxylic acid?
It's just another amide bond.
It's just another amide bond.
Shouldn't it terminate with a COOH at the end?
The amide bond between Gly and Leu is formed by the NH2 of Gly and the COOH to become NH-CO, so the other end of Gly should terminate with COOH?
The structure of Glycine is NH2-CH2-COOH but the image suggests it's NH2-CO-CH2-NH2 unless there is some other etiquette for terminating a structure with a weird amide bond?
Original post by Allergy
Shouldn't it terminate with a COOH at the end?
The amide bond between Gly and Leu is formed by the NH2 of Gly and the COOH to become NH-CO, so the other end of Gly should terminate with COOH?
The structure of Glycine is NH2-CH2-COOH but the image suggests it's NH2-CO-CH2-NH2 unless there is some other etiquette for terminating a structure with a weird amide bond?
The amide bond between Gly and Leu is formed by the NH2 of Gly and the COOH to become NH-CO, so the other end of Gly should terminate with COOH?
The structure of Glycine is NH2-CH2-COOH but the image suggests it's NH2-CO-CH2-NH2 unless there is some other etiquette for terminating a structure with a weird amide bond?
The glycine has formed another amide bond.
Quick Reply
Related discussions
- Chemistry vs chemical engineering
- DNA multiple choice question
- My dad doesn’t wash his hands after pouring bleach
- women sizing
- Are you Depressed, Anxious, Stressed ?.
- Is materials relevant to chemical engineering?
- Ranking all engineering fields
- Apply for Finance/Banking internship whilst studying chemistry
- Help urgent spectroscopy
- Personal statement help for chemistry
- What is engineering like?
- Admission rate for Chemical Engineering at Cambridge; Why do they vary so much?
- Chemical engineering from chemistry
- What type of isomerism do Ketones and Functional groups have?
- Wolverhampton or Roehampton for Psychology Conversion Msc?
- NHS trust says transgender milk just as good for babies as normal milk
- Physics sankey diagram question
- Undergraduate: What is the difference between Mechanical and General Engineering?
- give me 1 reason how video games are useful
- Chemistry personal statement
Latest
Last reply 1 minute ago
Official University of St Andrews Applicant Thread for 2024Last reply 10 minutes ago
Official Veterinary Medicine Applicants thread 2024 entryLast reply 19 minutes ago
BAE systems degree apprenticeships September 2024Last reply 41 minutes ago
What is the difference between ejusdem generis and noscitur a sociis?Last reply 41 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 43 minutes ago
Risus eget quam volutpat nisl in. Posuere mauris quam quis interdum vestibulum. Sed pLast reply 47 minutes ago
Durham vs Exeter Finance/AccountingLast reply 51 minutes ago
British Airways Apprenticeships 2024Trending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these products



