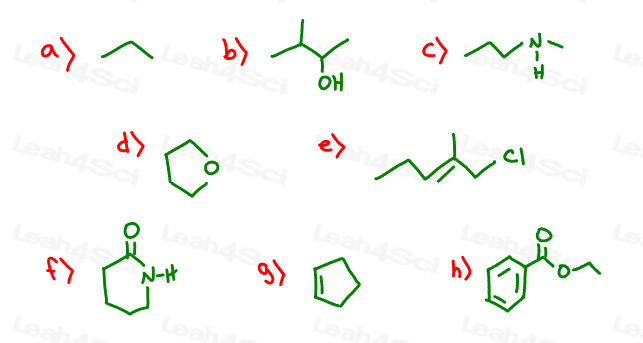
AS Chemistry organic help URGENT!!!
Scroll to see replies
The double lines in e, g and h, what do they also mean?
zig zag = straight chain compound
hexagon = cyclic with 6 carbons in the main chain
double line = double bond
hexagon = cyclic with 6 carbons in the main chain
double line = double bond
Original post by Divergent4
zig zag = straight chain compound
hexagon = cyclic with 6 carbons in the main chain
double line = double bond
hexagon = cyclic with 6 carbons in the main chain
double line = double bond
But why has B) got lines coming out of it and c) is a zig-zag when neither have a cyclic shape?
For e.g.

the second diagram, it has not got a cyclic shape but looks like a hexagon, why can't it be a zig-zag shape?
the second diagram, it has not got a cyclic shape but looks like a hexagon, why can't it be a zig-zag shape?
B has a functional group 'OH' which has to be expressed with a line out and OH written for representing a alcohol.
Skeletal formula is a way or representing the structure of an organic molecule. I doesn't have any symbols (e.g C for carbon, or H for hydrogen). Each straight line represents a carbon- carbon single bond, so it represents a carbon atom at each corner of the zigzag as well as the two ends of the zig zag. Each carbon atom is assumed to have 4 bonds in total which is why hydrogen isn't shown in the skeletal formula. In an organic compound each carbon atom may bond to another carbon atom (or two) so hydrogen atoms bond to the carbon atoms to make sure that each carbon atom has four bonds. Essentially, the zig zag is the carbon backbone of an organic molecule and the hydrogen atoms fill in the spaces.
The double line represents a carbon carbon double bond.
The hexagon is a ring of carbons. It's the same principle as before except every carbon atom is bonded to at least two other carbon atoms. Hydrogen atoms bond to the carbon atoms to make sure that each carbon atom is bonded to four atoms (like before).
The way I did it was at every end and corner of the zig zag, I would put a C, for carbon. I would then fill in the remained carbon-hydrogen bonds to make it up to four bonds for each carbon atom.
The double line represents a carbon carbon double bond.
The hexagon is a ring of carbons. It's the same principle as before except every carbon atom is bonded to at least two other carbon atoms. Hydrogen atoms bond to the carbon atoms to make sure that each carbon atom is bonded to four atoms (like before).
The way I did it was at every end and corner of the zig zag, I would put a C, for carbon. I would then fill in the remained carbon-hydrogen bonds to make it up to four bonds for each carbon atom.
if you see in the pic u posted...there are two lines and a chlorine functional group sticking out in the middle. the middle is a carbon and the end of those two lines are also a carbon each in which 3 hydrogens can attach as carbon makes 4 bonds.
each line sticking out when its not in a zigzag is a functional group e.g. alkyl/alchol/halogen etc
when theres a line sticking out of the zigzag and its not labelled its an alkyl (methyl to be precise) group and say if theres two lines coming from the zigzag its an ethyl group and three lines is a propyl group and so on as the carbon stem number increases u label as per...
Original post by fullmetal heart
Skeletal formula is a way or representing the structure of an organic molecule. I doesn't have any symbols (e.g C for carbon, or H for hydrogen). Each straight line represents a carbon- carbon single bond, so it represents a carbon atom at each corner of the zigzag as well as the two ends of the zig zag. Each carbon atom is assumed to have 4 bonds in total which is why hydrogen isn't shown in the skeletal formula. In an organic compound each carbon atom may bond to another carbon atom (or two) so hydrogen atoms bond to the carbon atoms to make sure that each carbon atom has four bonds. Essentially, the zig zag is the carbon backbone of an organic molecule and the hydrogen atoms fill in the spaces.
The double line represents a carbon carbon double bond.
The hexagon is a ring of carbons. It's the same principle as before except every carbon atom is bonded to at least two other carbon atoms. Hydrogen atoms bond to the carbon atoms to make sure that each carbon atom is bonded to four atoms (like before).
The way I did it was at every end and corner of the zig zag, I would put a C, for carbon. I would then fill in the remained carbon-hydrogen bonds to make it up to four bonds for each carbon atom.
The double line represents a carbon carbon double bond.
The hexagon is a ring of carbons. It's the same principle as before except every carbon atom is bonded to at least two other carbon atoms. Hydrogen atoms bond to the carbon atoms to make sure that each carbon atom is bonded to four atoms (like before).
The way I did it was at every end and corner of the zig zag, I would put a C, for carbon. I would then fill in the remained carbon-hydrogen bonds to make it up to four bonds for each carbon atom.
in the picture of skeletal formula for organic compounds i posted, in the second diagram could I have also drawn it like this:
Original post by GCSE 9
in the picture of skeletal formula for organic compounds i posted, in the second diagram could I have also drawn it like this:

Im not sure if it's technically correct but I think I provides the same information. You should check with your teacher.
Ideally I think you're supposed to spread out the branches as much as possible like the diagram and this
They have the same angles between each branch (120 degrees each, yours has 180, 90 and 90 degrees).
Original post by GCSE 9
Zig-zag = straight chain, for example linear pentane (C5H12).
Circle/hexagon type shape = cyclic compounds like cyclohexane. You'll learn more about those later on so don't panic!
The double line means a double bond C=C, rather than C-C. This is where two pairs of electrons are being shared instead of one.
Original post by fullmetal heart
Im not sure if it's technically correct but I think I provides the same information. You should check with your teacher.
Ideally I think you're supposed to spread out the branches as much as possible like the diagram and this

They have the same angles between each branch (120 degrees each, yours has 180, 90 and 90 degrees).
Ideally I think you're supposed to spread out the branches as much as possible like the diagram and this
They have the same angles between each branch (120 degrees each, yours has 180, 90 and 90 degrees).
But in the picture- diagram 2 (to the right) it was different from both of ours:
Their was a kind of hexagon shape, so which shape is correct

Original post by LRxS
Zig-zag = straight chain, for example linear pentane (C5H12).
Circle/hexagon type shape = cyclic compounds like cyclohexane. You'll learn more about those later on so don't panic!
The double line means a double bond C=C, rather than C-C. This is where two pairs of electrons are being shared instead of one.
Circle/hexagon type shape = cyclic compounds like cyclohexane. You'll learn more about those later on so don't panic!
The double line means a double bond C=C, rather than C-C. This is where two pairs of electrons are being shared instead of one.
Thanks, but in the picture i posted the shape was kind of a hexagon (2nd skeletal shape) but it wasn't cyclic???
The straight chain diagrams aren't actually correct for how the compounds are portrayed in real life. The zig zags and various not straight diagrams are correct for real life, the straight ones are just there to express what they contain really. It's because of the charge on the atoms, their electron layers repel the layers on another atom and so they lay in a way that keeps them furthest away from each other essentially.
Original post by GCSE 9
Thanks, but in the picture i posted the shape was kind of a hexagon (2nd skeletal shape) but it wasn't cyclic???
Ahhh sorry, I can't load the photo for some reason so I guessed 😂 I'll try and load it.
Every carbon has to be a corner or the end of a line.
@LRxS on your cyclopropane, you should draw the hydrogens outside the ring in the displayed formula.
Quick Reply
Related discussions
- chemistry alevel
- Organic chemistry textbook (uni)
- A level chemistry for a biology degree? (help please!!)
- Chemistry revision
- How can I become obsessed with chemistry ??
- Learning techniques
- AS/A Level Chemistry Study Group 2023/2024
- Chemistry at Uni?
- Biochem to Med chem
- Inorganic Chemistry (AQA) - A level
- a level biology and chemustry
- OCR A Chemistry Predictions
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me?
- Does anyone have a great summary sheet for organic chemistry?
- Organic, Physical or Inorganic Chemistry for tutoring?
- should i give up?
- How to increase grade in chemistry
- aqa a level chemistry paper 2 assessed topics
- As level chemistry paper 1
- Chem alevel
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products