[A Level] Monochloro Isomers
Hello everyone, hope we're all having a wonderful day.
I've already sat the exam but I've come across this question and can't let it go...
Here is a screenshot of the question in question: ( ...wait... )
)
I've put some annotations on the diagram so it can be easily referred to later.
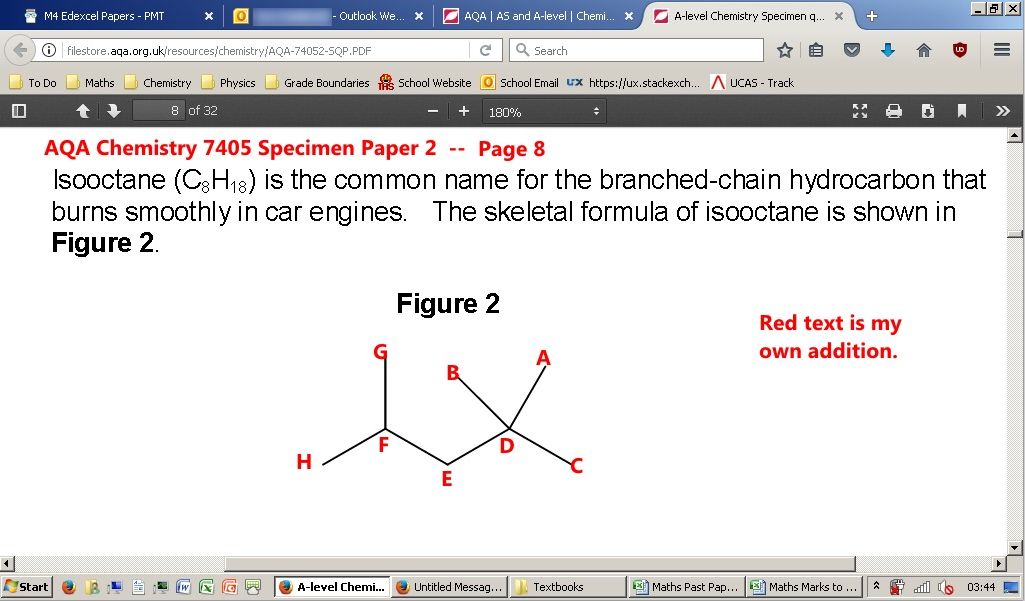
Question 03.5 (not shown) states: "Deduce the number of monochloro isomers formed by isooctane. Draw the structute of the monochloro isomer that exists as a pair of optical isomers."
I got the structure fine but I got the wrong number of monochloro isomers. The mark scheme says 4 but I obtained 5 as follows:
1) A chlorine atom replaces a hydrogen atom on A or B or C
2) A chlorine atom replaces a hydrogen atom on F
3) A chlorine atom replaces a hydrogen atom on G or H
4 & 5) If a chlorine atom replaces one of the hydrogen atoms on carbon E, then carbon E will become chiral. So this accounts for two isomers.
I suspect the problem lies with my logic for carbon E but I would appreciate it if anyone could check me here.
For reference, here is a link to the paper:
http://filestore.aqa.org.uk/resources/chemistry/AQA-74052-SQP.PDF (Question 03.5 on page 8/9)
Thanks in advance!
I've already sat the exam but I've come across this question and can't let it go...
Here is a screenshot of the question in question: ( ...wait...
 )
)I've put some annotations on the diagram so it can be easily referred to later.

Question 03.5 (not shown) states: "Deduce the number of monochloro isomers formed by isooctane. Draw the structute of the monochloro isomer that exists as a pair of optical isomers."
I got the structure fine but I got the wrong number of monochloro isomers. The mark scheme says 4 but I obtained 5 as follows:
1) A chlorine atom replaces a hydrogen atom on A or B or C
2) A chlorine atom replaces a hydrogen atom on F
3) A chlorine atom replaces a hydrogen atom on G or H
4 & 5) If a chlorine atom replaces one of the hydrogen atoms on carbon E, then carbon E will become chiral. So this accounts for two isomers.
I suspect the problem lies with my logic for carbon E but I would appreciate it if anyone could check me here.
For reference, here is a link to the paper:
http://filestore.aqa.org.uk/resources/chemistry/AQA-74052-SQP.PDF (Question 03.5 on page 8/9)
Thanks in advance!
(edited 5 years ago)
Original post by Tommy59375
Hello everyone, hope we're all having a wonderful day.
I've already sat the exam but I've come across this question and can't let it go...
Here is a screenshot of the question in question: ( ...wait... )
)
I've put some annotations on the diagram so it can be easily referred to later.

Question 03.5 (not shown) states: "Deduce the number of monochloro isomers formed by isooctane. Draw the structute of the monochloro isomer that exists as a pair of optical isomers."
I got the structure fine but I got the wrong number of monochloro isomers. The mark scheme says 4 but I obtained 5 as follows:
1) A chlorine atom replaces a hydrogen atom on A or B or C
2) A chlorine atom replaces a hydrogen atom on F
3) A chlorine atom replaces a hydrogen atom on G or H
4 & 5) If a chlorine atom replaces one of the hydrogen atoms on carbon E, then carbon E will become chiral. So this accounts for two isomers.
I suspect the problem lies with my logic for carbon E but I would appreciate it if anyone could check me here.
For reference, here is a link to the paper:
http://filestore.aqa.org.uk/resources/chemistry/AQA-74052-SQP.PDF (Question 03.5 on page 8/9)
Thanks in advance!
I've already sat the exam but I've come across this question and can't let it go...
Here is a screenshot of the question in question: ( ...wait...
 )
) I've put some annotations on the diagram so it can be easily referred to later.

Question 03.5 (not shown) states: "Deduce the number of monochloro isomers formed by isooctane. Draw the structute of the monochloro isomer that exists as a pair of optical isomers."
I got the structure fine but I got the wrong number of monochloro isomers. The mark scheme says 4 but I obtained 5 as follows:
1) A chlorine atom replaces a hydrogen atom on A or B or C
2) A chlorine atom replaces a hydrogen atom on F
3) A chlorine atom replaces a hydrogen atom on G or H
4 & 5) If a chlorine atom replaces one of the hydrogen atoms on carbon E, then carbon E will become chiral. So this accounts for two isomers.
I suspect the problem lies with my logic for carbon E but I would appreciate it if anyone could check me here.
For reference, here is a link to the paper:
http://filestore.aqa.org.uk/resources/chemistry/AQA-74052-SQP.PDF (Question 03.5 on page 8/9)
Thanks in advance!
A chlorine atom replacing a hydrogen atom on G or H gives two optical isomers as F becomes chiral.
Quick Reply
Related discussions
- A level Chemistry help
- isomerism a level chemistry urgent help
- A level Chemistry OCR HELP
- Complex ions and optical isomer question help please :)
- Why is 1,1,2-trimethylpropane not an isomer of hexane
- Isomers
- Another a level chem question that makes zero sense, help please!
- Help on a question regarding the stereoisomerism of a cyclic compound.
- Drawing isomers -Edexcel IGCSE chemistry
- AQA A Level Biology- Amylose and Amylopectin.
- Reaction Products
- Suggest a curved arrow mechanism to account for the thermal rearrangement of the ally
- Isomerism
- chemistry question!
- Unit 14: Applications of Organic Chemistry
- GCSE Chemistry Past Paper Question
- chem isomer as help?
- iupac naming of chromanones
- HELP edexcel a level chem
- Complex ions showing optical isomerism question
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



