This discussion is now closed.
Check out other Related discussions
- ocr a level chemistry
- Chemistry help on benzene
- Bad interview-pharmacy
- Chemistry - Benzene
- Chemistry - organic synthesis
- Alevel Chemistry Aromatic Compounds
- A level chemistry question
- Spectroscopy help
- Connectivity chem
- Chemistry UV-vis spectrophotometer problem
- AQA chem polymers quick question
- Benzene
- Chem Multiple choice % yield Q!
- Make it More Hydrocarbon-ey !!
- chemistry help
- benzene project
- Electrophillic/nucelophillic substitution /addition
- A-Level Chemistry (CAIE)
- Any Chemistry Mass Spectrum nerds help !!!!!!
- A Level Chemistry Organic Help
Benzenes - Kekule and stability
Hi,
Can someone explain this business with Arenes, about how Benzenes can become Kekule benzenes?
I don't understand the stability and how it's represented on the ethalpy diagram.
Thanks.
Can someone explain this business with Arenes, about how Benzenes can become Kekule benzenes?
I don't understand the stability and how it's represented on the ethalpy diagram.
Thanks.
Scroll to see replies
arenes such as bezene have a conjugated (joined up) pi system, kekule incorrectly theorized that benzene had alternating double bonds, so his was just his model. So theres no such thing as a 'kekule benzene' and although its often drawn with 3 single and 3 double bonds, the bond lengths are all equal
the delocalised benzene is more stable than kekules model predicted, the 'real' benzene the one with a delocalised structure is definatly lower than kekules model on an enthalpy profile
Kekules model is an actual substance its 1,3,5 cyclo hexene. the higher enthalpy profile
is caused by greater stability in the molecule and an increase in bonds to be over come.
is caused by greater stability in the molecule and an increase in bonds to be over come.
Kekule structure consisted alternative single and double bonds, which switched.
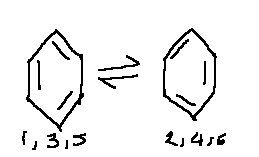
This was proved to be incorrect as,
- it should undergo electrophilic addition- but kekule's structure didn't
- x ray diffraction showed equal electron density around each carbon, indicating all the bonds are the same length, and the length being halfway between a single and a double bond.
Benzene is more stable as each carbon uses one of its electrons to for a pi bond, which are delocalised around the whole ring, making it more stable than other alkenes.
That's all I can remember right now, hope its helpful in some way.

This was proved to be incorrect as,
- it should undergo electrophilic addition- but kekule's structure didn't
- x ray diffraction showed equal electron density around each carbon, indicating all the bonds are the same length, and the length being halfway between a single and a double bond.
Benzene is more stable as each carbon uses one of its electrons to for a pi bond, which are delocalised around the whole ring, making it more stable than other alkenes.
That's all I can remember right now, hope its helpful in some way.
forgottenromeo
Kekules model is an actual substance its 1,3,5 cyclo hexene. the higher enthalpy profile
is caused by greater stability in the molecule and an increase in bonds to be over come.
is caused by greater stability in the molecule and an increase in bonds to be over come.
no. there is no substance with the structure that kekule predicted. Also if something is higher on an energy profile diagram, it is more unstable
Kekule had a dream about a snake eating its tail ... I hope this is useful in some way
charco
Kekule had a dream about a snake eating its tail ... I hope this is useful in some way
haha

The kekule structure is feasible as its found in penicillins and when they break down it reacts with hydrogen to form it, its just not the structure of benzene.
forgottenromeo
The kekule structure is feasible as its found in penicillins and when they break down it reacts with hydrogen to form it, its just not the structure of benzene.
Trust me its not a real structure, penicillins mode of action is to do with the beta-lactam ring, the aromatic group is just a side chain variant. There are other known C6H6 structures, but cyclohexatriene isn't one of them

... There is no reason for it not to exsist, and by experiment it can be proved to exsist. I said nothing about the mode of action of penicillin i just said that the triple double bonds in a cyclo hexene exsist in it
forgottenromeo
... There is no reason for it not to exsist, and by experiment it can be proved to exsist. I said nothing about the mode of action of penicillin i just said that the triple double bonds in a cyclo hexene exsist in it
Best of luck

EierVonSatan
Best of luck 


forgottenromeo
... There is no reason for it not to exsist, and by experiment it can be proved to exsist. I said nothing about the mode of action of penicillin i just said that the triple double bonds in a cyclo hexene exsist in it
it doesn't seem like you're quite getting the difference between 3 double bonds, which is perfectly possible, and 3 ALTERNATING double bonds, which...just...doesn't happen.
Its plausible though, if you look at certain mechanisms for reactions double bonds breaking and reforming does happen.
no...it's...really not. Bonds don't just break and reform alternatingly for no reason. that's not how it works.
And if the substance was in a vacuum then there would be no reason for it to happen, but it is also under the effect of various radiations. Not to mention the entire reason they are called electron clouds.
I think you're a bit confused, forgottenromeo. I'll post the bit in my Chemical Ideas book (the Salters syllabus book) where it talks about the Kekule structure...

You can think of the delocalised structure of benzene as halfway between the two extreme structures:

These are sometimes called Kekule structures because they were first proposed by August Kekule in 1865. Neither of these forms actually exists, and the delocalised arrangement is often represented by drawing a circle inside the ring.

These are sometimes called Kekule structures because they were first proposed by August Kekule in 1865. Neither of these forms actually exists, and the delocalised arrangement is often represented by drawing a circle inside the ring.

You can't rely on texts books, some of the things in them are wrong but we still have to answer in some of the cases, such as the exsistance of mesosomes in prokaryotic cells.
Related discussions
- ocr a level chemistry
- Chemistry help on benzene
- Bad interview-pharmacy
- Chemistry - Benzene
- Chemistry - organic synthesis
- Alevel Chemistry Aromatic Compounds
- A level chemistry question
- Spectroscopy help
- Connectivity chem
- Chemistry UV-vis spectrophotometer problem
- AQA chem polymers quick question
- Benzene
- Chem Multiple choice % yield Q!
- Make it More Hydrocarbon-ey !!
- chemistry help
- benzene project
- Electrophillic/nucelophillic substitution /addition
- A-Level Chemistry (CAIE)
- Any Chemistry Mass Spectrum nerds help !!!!!!
- A Level Chemistry Organic Help
Latest
Last reply 1 minute ago
Feeling inferior compared to syrians as a half moroccan/half whiteLast reply 1 minute ago
Telecommunications engineer job with "Provisional" licence?Last reply 2 minutes ago
Edexcel A-level French Paper 3, IRP/Speaking (9FR0 03) - 2024 [Exam Chat]Last reply 2 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 7 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2867
Last reply 8 minutes ago
can't go to ucl bc intl fees but got home fees for others :(Last reply 9 minutes ago
Woodhouse College applicants 2024Last reply 11 minutes ago
Customer Services Group - Operational Delivery - Administrative OfficersLast reply 13 minutes ago
St dominics, henrietta barnett, RMS, parmiters or girls grammar??Last reply 16 minutes ago
LSE anthropology and law 2024Posted 16 minutes ago
Can I get funding for a full time course if I've studied before?Last reply 17 minutes ago
Degree Apprenticeship at Accenture (assessment centre)Posted 17 minutes ago
Durham vs Exeter Finance/AccountingLast reply 20 minutes ago
Customer Services Group; Executive Officer (EO) - Home Office - Various Roles

