Chemistry help: electron density maps
I am a bit confused as to why in electron density maps for ionic compounds, there is no single line representing electron density that surrounds both cations and anions. When I look at diagrams of electron density, I can see single lines that surround both the cation and anion, so I am not sure what I am missing.
Original post by ahow39409234-095
I am a bit confused as to why in electron density maps for ionic compounds, there is no single line representing electron density that surrounds both cations and anions. When I look at diagrams of electron density, I can see single lines that surround both the cation and anion, so I am not sure what I am missing.
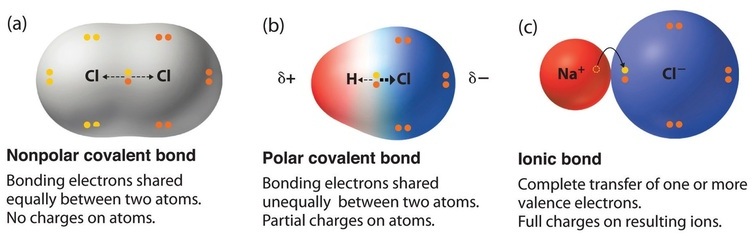
From what I understand, there wouldn't be much significant overlap between orbitals in an ionic compound because an ionic bond is very polar. In a covalent bond, the electrons are shared between atoms due to the orbital overlap, whereas in an ionic compound, an electron is "transferred from one atom to another resulting in ions held together by an electrostatic attraction
Sorry, I accidently sent my other post before I managed to finish it!
What I was trying to say was, perhaps the orbital overlap in ionic bonding is not significant enough to be shown in an electron density map? There is still some overlap, but just not as much as in covalent bonding. Perhaps it depends on the difference in electronegativities.
Could you post an image of some of the maps you are looking at?
What I was trying to say was, perhaps the orbital overlap in ionic bonding is not significant enough to be shown in an electron density map? There is still some overlap, but just not as much as in covalent bonding. Perhaps it depends on the difference in electronegativities.
Could you post an image of some of the maps you are looking at?
Think of the lines like contours on a map, but instead of showing height, the lines are showing increasing electron density - the map for NaCl looks like a series of concentric circles, the inner circles have higher electron density than the outer circles. Please see attached picture
Quick Reply
Related discussions
- Dissociation chemistry
- AQA A Level Chemistry
- Chemistry - acids
- dipole dipole vs vanderwaals forces difference
- I have no clue to this chemistry 1 marker
- Can someone explain to me charge density, a level chemistry
- Drift Velocity Q
- Isaac Physics - Drift Velocity (A level)
- how do u know when to say ions/electrons
- Physics homework help
- a level chemistry drawing moelceules
- A level physics help
- Help easy stats q
- factors effecting bond strength
- Help urgent spectroscopy
- UKChO 2020 Q6 help
- how do you identify permanent dipole- permanent dipole molecules?
- Magnesium melting point
- Can someone explain why the type of spectrum depends on the temperature of the atmosp
- OCR Biology Help:
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



