This discussion is now closed.
Check out other Related discussions
- Random OCR A-level organic mechanism reactions paper 3
- AS/A Level Chemistry Study Group 2023/2024
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- Organic chemistry question
- Help chemistry urgent
- chemistry question
- A-level Chemistry Study Group 2022-2023
- Chemistry question organic
- Dissociation chemistry
- Chemistry - acids
- Magnesium melting point
- A-Level chemistry
- Hydrolysis
- Chemistry help urgent Alevel
- SN1 and SN2 reactions (chemistry)
- A level chemistry optical isomerism MC questions
- a level chemistry drawing moelceules
- Help chem
- chemistry
- Ask Us Anything!
OCR Chemistry A/S Unit One - Atoms, Bonds and Groups
Hey there, I've looked around on this and there's no specific thread for this exam so I thought I'd create one! Hope everyone's okay with revision! If anyone's reading this, Can you explain what a polar molecule is? and can a molecule have 'polar bonds' but not be a polar molecule?
Fatema
Fatema
Scroll to see replies
This may be wrong but from my knowledge a Polar Molecule is a bit like a magnet, one end had a postive charge and one end has a negative charge, this means it can attract other molecules, i think there isn't really anything called a 'Polar Bond' but I know what you mean, but if the bonds are polar then the molecule will be polar
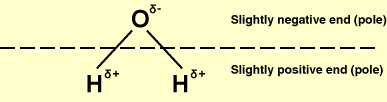
I may be wrong though so anyone who reads this please correct me if I am wrong.
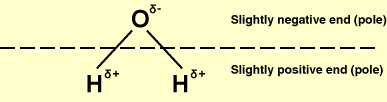
I may be wrong though so anyone who reads this please correct me if I am wrong.
Fatema991
Hey there, I've looked around on this and there's no specific thread for this exam so I thought I'd create one! Hope everyone's okay with revision! If anyone's reading this, Can you explain what a polar molecule is? and can a molecule have 'polar bonds' but not be a polar molecule?
Fatema
Fatema
A polar molecule is formed when there is an uneven distribution of electrons across a molecule (i.e. some atoms are electronegative which means that they drawing the binding electrons towards themselves, thus giving themselves a partial negative charge and the other atom in the bond a partial positive charge)
When the partially positive end of one molecule is near the partially negative end of another molecule, they attract one another (much like magnets) and the mutual attraction is known as EDIT: Van der Waal's forces (EDIT: therefore it is only possible with molecules containing polar bonds)
Hope this helps

Adhavan
A polar molecule is formed when there is an uneven distribution of electrons across a molecule (i.e. some atoms are electronegative which means that they drawing the binding electrons towards themselves, thus giving themselves a partial negative charge and the other atom in the bond a partial positive charge)
When the partially positive end of one molecule is near the partially negative end of another molecule, they attract one another (much like magnets) and this mutual attraction is known as a polar bond (therefore it is only possible with polar molecules)
Hope this helps
When the partially positive end of one molecule is near the partially negative end of another molecule, they attract one another (much like magnets) and this mutual attraction is known as a polar bond (therefore it is only possible with polar molecules)
Hope this helps

You can get polar bonds in non-polar molecules, eg, a linear molecule.
EDIT: Lol looking at your AS marks, I'm probably wrong.

Hmm actually, I think what you have described as a polar bond is actually an intermolecular force.
Is this AS Salters Chemistry (new for 2009)? Cos I'm sure this doesnt come up at all.
DaveJ
You can get polar bonds in non-polar molecules, eg, a linear molecule.
EDIT: Lol looking at your AS marks, I'm probably wrong.
EDIT: Lol looking at your AS marks, I'm probably wrong.

Care to elaborate?
DaveJ
You can get polar bonds in non-polar molecules, eg, a linear molecule.
EDIT: Lol looking at your AS marks, I'm probably wrong.
Hmm actually, I think what you have described as a polar bond is actually an intermolecular force.
EDIT: Lol looking at your AS marks, I'm probably wrong.

Hmm actually, I think what you have described as a polar bond is actually an intermolecular force.
Bond polarity has nothing to do with molecular shape.
trance addict
Oh and a molecule can have polar bonds but not be a polar molecule when the atoms bonding to each other have a similiar electronegativity, the charges cancel each other out and the molecule has no overall charge (think of H2) so there is no delta negative/positive charge on either side of the molecule to attract and bond with other molecuels via permanent dipole-dipole forces (they can still bond via van der waals forces though)
Oh and a molecule can have polar bonds but not be a polar molecule when the atoms bonding to each other have a similiar electronegativity, the charges cancel each other out and the molecule has no overall charge (think of H2) so there is no delta negative/positive charge on either side of the molecule to attract and bond with other molecuels via permanent dipole-dipole forces (they can still bond via van der waals forces though)
Forgot to mention this

Molecules can have an induced charge and bond via temporary dipole-induced dipole forces (although technically when a dipole is temporary/induced, the molecule is polar at that point)
Adhavan
Forgot to mention this 
Molecules can have an induced charge and bond via temporary dipole-induced dipole forces (although technically when a dipole is temporary/induced, the molecule is polar at that point)

Molecules can have an induced charge and bond via temporary dipole-induced dipole forces (although technically when a dipole is temporary/induced, the molecule is polar at that point)
those are van der waals forces :P thats dipole induction when the two repelling forces induce a temporary dipole
Adhavan
Care to elaborate?
Well basically, I think in your post, what you described as a polar molecule is actually a polar bond, and what you described as a polar bond is actually an intermolecular force.
A polar bond is when for example 2 atoms bond covalently, and one is more electronegative than the other, thus the shared electrons are closer to that atom, creating one delta plus atom and one delta minus atom.
A polar molecule is whenever the delta plus and delta minus regions of the molecule do not cancel each other out, as the molecule is not symmetrical.
Tbh, I'm probably wrong.
trance addict
those are van der waals forces :P thats dipole induction when the two repelling forces induce a temporary dipole
lol no i meant that I forgot to mention what i quoted from you in my initial answer

Adhavan
A polar molecule is formed when there is an uneven distribution of electrons across a molecule (i.e. some atoms are electronegative which means that they drawing the binding electrons towards themselves, thus giving themselves a partial negative charge and the other atom in the bond a partial positive charge)
When the partially positive end of one molecule is near the partially negative end of another molecule, they attract one another (much like magnets) and this mutual attraction is known as a polar bond (therefore it is only possible with polar molecules)
Hope this helps
When the partially positive end of one molecule is near the partially negative end of another molecule, they attract one another (much like magnets) and this mutual attraction is known as a polar bond (therefore it is only possible with polar molecules)
Hope this helps

Generally, a polar bond refers to bond polarity, i.e. a covalent bond where there is a difference in electronegativity between the two atoms involved. For example H-Cl or C=O, No?
DaveJ
Well basically, I think in your post, what you described as a polar molecule is actually a polar bond, and what you described as a polar bond is actually an intermolecular force.
A polar bond is when for example 2 atoms bond covalently, and one is more electronegative than the other, thus the shared electrons are closer to that atom, creating one delta plus atom and one delta minus atom.
A polar molecule is whenever the delta plus and delta minus regions of the molecule do not cancel each other out, as the molecule is not symmetrical.
Hmm, an example off the top of my head about you saying polar bonds imply a polar molecule: HCl has a polar bond, but is linear.
A polar bond is when for example 2 atoms bond covalently, and one is more electronegative than the other, thus the shared electrons are closer to that atom, creating one delta plus atom and one delta minus atom.
A polar molecule is whenever the delta plus and delta minus regions of the molecule do not cancel each other out, as the molecule is not symmetrical.
Hmm, an example off the top of my head about you saying polar bonds imply a polar molecule: HCl has a polar bond, but is linear.
HCl is a polar molecule though
 are you sure you're not confusing yourself?
are you sure you're not confusing yourself?Toiletpaper8
Generally, a polar bond refers to bond polarity, i.e. a covalent bond where there is a difference in electronegativity between the two atoms involved. For example H-Cl or C=O, No?
yeah you're right - when i said polar bond i actually meant Van der Waal's forces

Sorry about that lol
DeanK2
Polar Molecule: There is a net permanent charge imbalance that has occured for this molecule.
Polar Bond: Can be found in non polar molecules Cf4, Ccl4, etc), where there is no symmetry. This is due to the elctronegativity difference between the atoms within the molecule.
Polar Bond: Can be found in non polar molecules Cf4, Ccl4, etc), where there is no symmetry. This is due to the elctronegativity difference between the atoms within the molecule.
Yes that is my point. The person I quoted said (I think) that polar bonds could only occur in polar molecules.
DeanK2
CF(4) is a non polar molecule, as overall there is no net charge imbalance, as the vector sum of each individual delta charge that has been created is zero.
I do, however, hope it is quite clear that the C-F are polar...
I do, however, hope it is quite clear that the C-F are polar...
again you're right - the problem is i was trying to explain this in simpler terms but ended up using terminology with entirely different meanings lol
when i said polar molecule initially, i actually meant any molecule containing polar bonds (although i probably should have said that to begin with)
Adhavan
HCl is a polar molecule though  are you sure you're not confusing yourself?
are you sure you're not confusing yourself?
 are you sure you're not confusing yourself?
are you sure you're not confusing yourself?What he's trying to say is that what you've described isn't a "polar bond", and he's right.
Conventionally speaking, H-Cl is a polar bond, C-F is a polar bond, C=O is a polar bond...
F delta minus and H delta plus isn't.
Plus, whilst a polar molecule has a polar bond, a molecule with a polar bond isn't necessarily polar.
Related discussions
- Random OCR A-level organic mechanism reactions paper 3
- AS/A Level Chemistry Study Group 2023/2024
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- Organic chemistry question
- Help chemistry urgent
- chemistry question
- A-level Chemistry Study Group 2022-2023
- Chemistry question organic
- Dissociation chemistry
- Chemistry - acids
- Magnesium melting point
- A-Level chemistry
- Hydrolysis
- Chemistry help urgent Alevel
- SN1 and SN2 reactions (chemistry)
- A level chemistry optical isomerism MC questions
- a level chemistry drawing moelceules
- Help chem
- chemistry
- Ask Us Anything!
Latest
Trending
Last reply 11 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 11 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



