Effect of temperature on electrochemical cells
Hi, I am doing a coursework on the effect of temperature and concentration of one of the electrolytes in an electrochemical cell. This cell is Zn/Cu cell. I don't have a problem with the concentration, but with temperature there seems to be a change of pd in the wrong direction, decreasing ( I am changing th ZnSO4 temp). According to Nernst equation, the pd should increase? Also, wouldn't this change be almost impossible to take readings of since the theoretical changes are very small? Also, are there other factors which would affect the readings as I have tried to read up on this over the internet, but there is limited information, such as resistance in the wire increases with temperature and thus more unreliable readings?
Many thanks in advance for the help
Luke
Many thanks in advance for the help
Luke
I'm actually doing the temperature part of this experiment tomorrow with a Daniell Cell. About the readings, our script says that they will be very small; to how many dp did you measure to?
For another part of the experiment we did change the concentration of the cells. The general errors we noticed that could arise were in the weighing out and making the CuSO4 and ZnSO4 solution, not cleaning electrodes properly; we had to use a digital multimeter to take our readings since the one on the power source was quite iffy, the solutions diffuse through the membrane so their concentrations decrease, etc.
I'll see what happens tomorrow and get back to you with results and some more errors. I'll talk to my lab partner, who's the clever one . How many repeats did you do?
. How many repeats did you do?
The emf changes seen will be very small, and, to avoid the effect being masked by diffusion across the membranes, one should aim to complete the experiment within 20 minutes.
For another part of the experiment we did change the concentration of the cells. The general errors we noticed that could arise were in the weighing out and making the CuSO4 and ZnSO4 solution, not cleaning electrodes properly; we had to use a digital multimeter to take our readings since the one on the power source was quite iffy, the solutions diffuse through the membrane so their concentrations decrease, etc.
I'll see what happens tomorrow and get back to you with results and some more errors. I'll talk to my lab partner, who's the clever one
 . How many repeats did you do?
. How many repeats did you do?I measured to four dp. Also, I used a salt bridge rather than a membrane. 3 repeats were done, all about the same.
So how did your experiment go?
Thanks
Luke
So how did your experiment go?
Thanks
Luke
As you increase the temperature, the pd should decrease looking at the Nernst equation. We ended up increasing the temperature of the whole system and as we increased the temperature, the pd decreased; vice versa. The change was quite small but you’re measuring to 4dp (as were we) so did you not see any change?
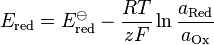
If you start off with a negative Eº value, as you increase the temperature your E value is going to get more negative so it's decreasing. But that's for one half-cell only.
Wait I just thought of something even more confusing than what I previously said
For Cu2+: Eº = +0.34V and it's being oxidised, Eº = -0.34V
For Zn2+: Eº = -0.76V and it's being reduced, Eº= -0.76V
So if you're Eº = -1.10V. You could look at the Nernst equation as being broken down into two bits – so your E = E(RHS, i.e. for Zn2+) – E(LHS, i.e. for Cu2+). So if you do RHS – LHS you get whatever value. The E value for copper should be positive overall because it’s - - = +. That zinc value is however going to be negative. As you increase the temperature the zinc value should get more and more negative. If Cu2+ is staying the same and Zn2+ is getting more negative, should your potential not decrease/get more negative? Or am I missing something

Thanks for the replies. I changed the temperature of just the zinc half cell whereas I kept the copper half cell at 298K. I thought that the zinc electrode is an anode and therefore if it were to become more negative, then the pd will increase since the copper cathode stays the same positive value?
I didn't think you could choose which was the anode and which is the cathode according to the electrochemical series where Zn2+ +2e- -> Zn = -0.76 and the copper one = +0.34..... I'm slightly confused
I didn't think you could choose which was the anode and which is the cathode according to the electrochemical series where Zn2+ +2e- -> Zn = -0.76 and the copper one = +0.34..... I'm slightly confused

No okay I’ve stuck my head in the sink, realised my notes were wrong and finally understood what’s going on.
The spontaneous reaction is: Cu2+ + Zn -> Cu + Zn2+ which would give you a positive value of Eº = +1.10V. However you can drive the non-spontaneous reaction Zn2+ + Cu -> Cu2+ + Zn which gives you a negative Eº = -1.10V.
Before I start talking rubbish again can I have the concentrations of your Cu2+ and Zn2+?
can I have the concentrations of your Cu2+ and Zn2+?
The spontaneous reaction is: Cu2+ + Zn -> Cu + Zn2+ which would give you a positive value of Eº = +1.10V. However you can drive the non-spontaneous reaction Zn2+ + Cu -> Cu2+ + Zn which gives you a negative Eº = -1.10V.
Before I start talking rubbish again
 can I have the concentrations of your Cu2+ and Zn2+?
can I have the concentrations of your Cu2+ and Zn2+?In the temperature change, the CuSO4 conc was 1.0 mol dm-3 and the ZnSO4 conc was 0.1 mol dm-3.
Thanks
Thanks
Original post by Rubs
I'm actually doing the temperature part of this experiment tomorrow with a Daniell Cell. About the readings, our script says that they will be very small; to how many dp did you measure to?
For another part of the experiment we did change the concentration of the cells. The general errors we noticed that could arise were in the weighing out and making the CuSO4 and ZnSO4 solution, not cleaning electrodes properly; we had to use a digital multimeter to take our readings since the one on the power source was quite iffy, the solutions diffuse through the membrane so their concentrations decrease, etc.
I'll see what happens tomorrow and get back to you with results and some more errors. I'll talk to my lab partner, who's the clever one . How many repeats did you do?
. How many repeats did you do?
For another part of the experiment we did change the concentration of the cells. The general errors we noticed that could arise were in the weighing out and making the CuSO4 and ZnSO4 solution, not cleaning electrodes properly; we had to use a digital multimeter to take our readings since the one on the power source was quite iffy, the solutions diffuse through the membrane so their concentrations decrease, etc.
I'll see what happens tomorrow and get back to you with results and some more errors. I'll talk to my lab partner, who's the clever one
 . How many repeats did you do?
. How many repeats did you do?Hey, so uncannily im doing the same experiment as you are, maybe with some differences, as i am changing temperatures and concentrations simultaneously providing me with multiple readings for a particular concentration. Kinda struggling here, any pointers or details i should look out for?
Quick Reply
Related discussions
- Chem a level - electrochemistry
- ocr a level chemistry
- pls help with my physics
- chemistry help
- Edexcel IGCSE Biology | PAPER 2
- AQA A Level Chemistry Electrochemistry
- OCR A-level chemistry practicals
- Chemistry
- Electrochemical cells AQA A Level chemistry
- Edexcel A Level Biology B Paper 2: 9BI0 02 - 17 Jun 2022 [Exam Chat]
- 25 mark essay question
- Viruses
- AQA A-Level Biology Paper 3 [21st June 2023] Exam Chat
- GCSE physics question circuits
- Kallisto's Saturday Question: Inventions
- AQA Biology essay
- biology help
- AQA A Level Chemistry Paper 3 7405/3 - 23 Jun 2022 [Exam Chat]
- Need Help on an Electrochem Q
- Kw Question A Level Chem
Latest
Last reply 4 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 8 minutes ago
Durham vs Exeter Finance/AccountingLast reply 14 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 15 minutes ago
Litigation Caseworker Home Office 2024Last reply 17 minutes ago
BT Degree Apprenticeship 2024Last reply 21 minutes ago
Application approved before evidence was able to be submittedLast reply 26 minutes ago
AQA Level 2 Further Maths 2024 Paper 1 (8365/1) - 11th June [Exam Chat]Last reply 28 minutes ago
March Riba part 2 Manchester vs SheffieldLast reply 42 minutes ago
Confused in undergrad final grade at University of GreenwichTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products


