Drawing shape of ions
'draw the shape of PO4 (-3) ( the -3 is the charge on the ion)'
So can you use the shape of PO4 and add 3 electrons to your dot and cross diagram?
So can you use the shape of PO4 and add 3 electrons to your dot and cross diagram?
Original post by indignation
'draw the shape of PO4 (-3) ( the -3 is the charge on the ion)'
So can you use the shape of PO4 and add 3 electrons to your dot and cross diagram?
So can you use the shape of PO4 and add 3 electrons to your dot and cross diagram?
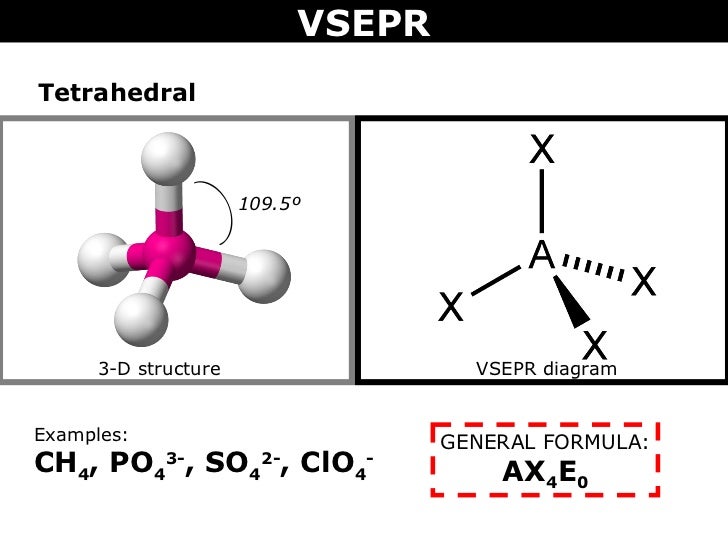
If you need the shape then you don`t need to do a dot and cross diagram. As there are 4 sets of electrons around the central phosphorus it will have a tetrahedral shape with 109 degree bond angles (this is for the OCR B Salters sylabus)
Note: There will be three single bonds to oxygen and one double bond.
(edited 7 years ago)
Original post by SamuelN98
If you need the shape then you don`t need to do a dot and cross diagram. As there are 4 sets of electrons around the central phosphorus it will have a tetrahedral shape with 109 degree bond angles (this is for the OCR B Salters sylabus)
Note: There will be three single bonds to oxygen and one double bond.

Note: There will be three single bonds to oxygen and one double bond.

How did you know about the 1 double bond and 2 single?
Wouldn't be 6 pairs of electrons, 4 bonding pairs and 2 lone pairs? Here's my thought process:
1/ Outer electrons of central atom P 5
2/ Electrons brought by the Oxygens 4x1
3/ Add 3 e- due to charge 12
4/ Divide by 2 6
Therefore wouldn't be a square planar?
1/ Outer electrons of central atom P 5
2/ Electrons brought by the Oxygens 4x1
3/ Add 3 e- due to charge 12
4/ Divide by 2 6
Therefore wouldn't be a square planar?
Original post by indignation
How did you know about the 1 double bond and 2 single?
This should help explain it.
http://terpconnect.umd.edu/~wbreslyn/chemistry/Lewis-Structures/lewis-structure-for-PO43-.html
Quick Reply
Related discussions
- Shapes of molecules
- Chemistry Questions
- a level chemistry drawing moelceules
- Complex ions showing optical isomerism question
- Have i explained how the Bohr effect can impact the structure of haemoglobin correct?
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- A level physics question
- GCSE Chemistry Past Paper Question
- Help chemistry alevel
- Chemistry ocr a level enthalpy
- Nitrate ion
- enthalpy change of sol
- How to re-attempt graph sketches in the GCSE.
- Will the bond angle in H2O(104.5) increase if water is bind to other molecules?
- AQA A Level Chemistry Paper 3 20th June 2018 Unofficial Markscheme
- Paper 3 AQA a Level biology
- Ideal and regular solutions question
- AS/A Level Chemistry Study Group 2023/2024
- Edexcel GCSE Chemistry Paper 1 Higher Tier 1CH0 1H - 27 May 2022 [Exam Chat]
- Atomic Structure - Shapes of S and P orbitals AQA a level chem
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



