Chemistry unit 4 AQA
Scroll to see replies
Original post by nasira372
How can you tell if the acid/base is weak or strong?
Also, how are we supposed to know what the salts of the weak acids/bases are?
Also, how are we supposed to know what the salts of the weak acids/bases are?
The acids: if it is not a common acid (one which you'd know whether it was strong or weak) then it will say in the question.
Any salt of A- will suffice.
Original post by thomas93
Original post by thomas93
I do, I do. That is for my 5 (now 4) exams though. And It's only because I genuinely enjoy the subjects, so I don't find it too painful.
I love organic, but only because the teacher I had for it was f*cking amazing! I know a lot of people really hate it, it must be like marmite. :P
I love organic, but only because the teacher I had for it was f*cking amazing! I know a lot of people really hate it, it must be like marmite. :P
you got any tips to revise organic?
especially N.M.R and carbon 13 spec
Original post by rommy123
http://www.mediafire.com/?r74x3pdjrhay7
^^^^ this might help you, you`ll have to download it, the notes are really good
^^^^ this might help you, you`ll have to download it, the notes are really good
They're actually awesome!
Original post by thomas93
Original post by thomas93
They're actually awesome!
yup they are

Original post by rommy123
you got any tips to revise organic?
especially N.M.R and carbon 13 spec
especially N.M.R and carbon 13 spec
Hmm, I think with those kind of topics practice makes perfect, because there isn't much knowledge on them, it's more the application of the knowledge. Just practice the, find as many NMR questions as you can and do them, over and over again if needs must.
Obviously make sure you're comfortable with the knowledge before you do that. And make sure you know the solvents and standard compounds, they're easy marks.
I know, it's a bit vague, but i hope that helps a little.

Original post by thomas93
Original post by thomas93
Hmm, I think with those kind of topics practice makes perfect, because there isn't much knowledge on them, it's more the application of the knowledge. Just practice the, find as many NMR questions as you can and do them, over and over again if needs must.
Obviously make sure you're comfortable with the knowledge before you do that. And make sure you know the solvents and standard compounds, they're easy marks.
I know, it's a bit vague, but i hope that helps a little.
Obviously make sure you're comfortable with the knowledge before you do that. And make sure you know the solvents and standard compounds, they're easy marks.
I know, it's a bit vague, but i hope that helps a little.

yup its all about practicing
 and thanks
and thanksIn naming compounds, do you need to know how to name phenyl alcohols?
or it limited to nitrobenzene, phenyl amine, phenyl ketones, phenylammonium salts?
or it limited to nitrobenzene, phenyl amine, phenyl ketones, phenylammonium salts?
Original post by Nuss
For making an alcohol via esterification, what are the conditions required?
Hydrolysis? Just a strong acid catalyst... It's an equilibrium reaction...
Original post by thomas93
Hydrolysis? Just a strong acid catalyst... It's an equilibrium reaction...
Are you meant to put reflux as one of the conditions? Saw a mark for it in one of the old past papers, but it was for making the ester as opposed to making an alcohol
(edited 12 years ago)
Original post by tgarrud
83/100 raw marks
The ums boundaries do not change; thats the whole point in them being scaled.
The ums boundaries do not change; thats the whole point in them being scaled.
Ah, sorry
 I've been working from some of the older past papers, pre-syllabus change. They're only out of 90.
I've been working from some of the older past papers, pre-syllabus change. They're only out of 90.Original post by Nuss
For making an alcohol via esterification, what are the conditions required?
Do you mean making an alcohol from an ester, or using an alcohol to form an ester? Conditions for esterification differ depending on what your using with the alcohol (acid chlorides react vigorously, so they can be used at room temperature; acid anhydrides less so, heat will be needed; carboxylic acids will require a sulphuric acid catalyst).
But if you want to get the alcohol from an ester, then you can go either base or acid catalysed hydrolysis. I think it's reflux for both of these (haven't yet seen a markscheme penalising 'reflux' so it doesn't hurt to add it on). Base-catalysed hydrolysis is also referred to as saponification (the salt you get can be made into soap).
(edited 12 years ago)
Original post by liviaaa
How much do we need to know about Chromatography? We have literally learnt about the moving/stationary phases and that it can be used to separate amino acids. Do we need to know about Rf values or anything like that? 

Know that separation by column chromatography depends
on the balance between solubility in the moving phase and
retention in the stationary phase.
Know that gas-liquid chromatography can be used to
separate mixtures of volatile liquid.
Original post by Erotas
Could somebody explain how to do 1aiv from the January 2011 paper please? Thanks 

You half each of the conc. in the rate equation (as water doubles the volume, so the conc of each halves), but k stays constant.
Original post by liviaaa
You half each of the conc. in the rate equation (as water doubles the volume, so the conc of each halves), but k stays constant.
Ohh right, that makes sense
 thank you.
thank you.Can someone help me with naming of amino acids? There was a question on JUN 11, but I don't have the paper on me to know which question number it as.
Original post by JesusIsMyHomeboy
Can someone help me with naming of amino acids? There was a question on JUN 11, but I don't have the paper on me to know which question number it as.
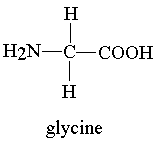
That is glycine. IUPAC name is 2-aminoethanoic acid.
Amino acid always has a carboxylic acid group at the end so will always end with ...oic acid
They also always have an amino group so will always start with x-amino....(the x is the number on the carbon where the amino group is).
Then it is just a matter of finding the shortest chain.
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- GCSE Exam Discussions 2023
- Oxford AQA International A-Level past papers
- How do you get A's in Biology A levels
- chemistry aqa a-level question help
- AS/A Level Chemistry Study Group 2023/2024
- My GCSE journey!! 🦞
- Life and Health science (step up science equivalent)
- oxfordAQA chemistry papers
- A-level Biology Study Group 2023-2024
- PG online computer science test answers
- applied science EXTENDED CERTIFICATE
- I'm confused about my As levels
- a level study : BLOG
- btec applied science extended certificate aqa
- D in chemistry As Level ....
- GCSE Results: Post your results
Latest
Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



