AQA CHEM1: 15th May 2012
Scroll to see replies
Original post by . .
They don't. I haven't seen any questions on the current spec where they ask you to draw orbitals.
I'm not talking about drawing orbitals. I'm talking about drawing 3d ionic lattices..here you go http://store.aqa.org.uk/qual/gce/pdf/AQA-CHEM1-W-QP-JUN09.PDF and if you look at the mark scheme, you get a mark for drawing the structure in 3D.
Original post by Philippamalko
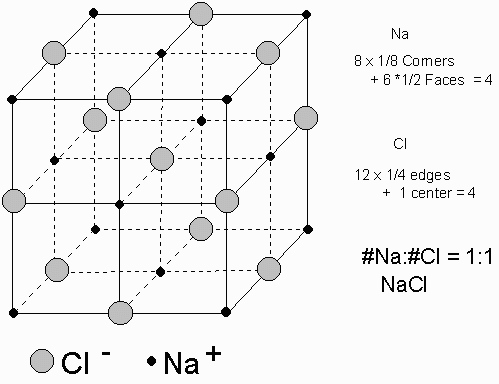
you'd draw rows of circles (your positive and negative ions) each with an alternating positive and negative charge. As if you were drawing a 3d cube, but made of circles, and stick a charge in each of them.
so the charges go + - + - + - + -
so the charges go + - + - + - + -
Fine, I get that, but what I'm having trouble with is actually drawing it in 3D..every time I attempt to draw a cube of circles, I always end up mucking it up. I'm pretty useless at art you see..

(edited 11 years ago)
Original post by Mystic Creature
I'm not talking about drawing orbitals. I'm talking about drawing 3d ionic lattices..here you go http://store.aqa.org.uk/qual/gce/pdf/AQA-CHEM1-W-QP-JUN09.PDF and if you look at the mark scheme, you get a mark for drawing the structure in 3D.
Sorry my mistake.
Original post by ambrin ox
Im really sorry but i didnt get that about the p orbitals? :/ pls help?

Alright, due to the repulsion caused by electron pairs in an orbital, electrons first fill up empty orbitals. Only once all the orbitals have at least one electron in do they begin to double up. This explains the drop in ionisation energy between group 5 and 6, because of the repulsion between the two electrons in the p-orbital

Original post by . .
Sorry my mistake.


Ahh okay, but do you have to draw the 'interior' atoms as well, or can we get away with just drawing all of the bonds from the 3 faces we can see (if you get what I mean lol)
Jan 2012 paper - can someone please explain 2d? (mark meltin point of nitrogen on graph with other group 2 elements on it)
Original post by Mystic Creature
Ahh okay, but do you have to draw the 'interior' atoms as well, or can we get away with just drawing all of the bonds from the 3 faces we can see (if you get what I mean lol)
Might as well you've got nothing to lose. I'm sure they are not going to penalise you for going into too much detail.

Original post by joker12345
Jan 2012 paper - can someone please explain 2d? (mark meltin point of nitrogen on graph with other group 2 elements on it)
I just realised that it was a gas and so had a low melting/boiling point (can't remember) but yeah, I wouldn't know how to know other than that. :B
@ below: Thanks, finally understand!
(edited 11 years ago)
Original post by joker12345
Jan 2012 paper - can someone please explain 2d? (mark meltin point of nitrogen on graph with other group 2 elements on it)
Nitrogen has a molecular structure as it is a non-metal. And molecular structures have low melting points because vdw forces exist between the molecules which don't require a lot of energy to overcome.
How do we know when to mention vdWs? For example, in Jan 11, Q5 (a) 'State the type of structure shown by a crystal of silicon.
Explain why the melting point of silicon is very high.'
I get that we say it is macromolecular with strong covalent bonds and need a lot of energy to break, but if we also say macromolecular so has lots of electrons and strong vdWs too, do we get 0 because mark scheme says "If IMF/H-bonds/Ionic/metallic CE =0/3"?
Explain why the melting point of silicon is very high.'
I get that we say it is macromolecular with strong covalent bonds and need a lot of energy to break, but if we also say macromolecular so has lots of electrons and strong vdWs too, do we get 0 because mark scheme says "If IMF/H-bonds/Ionic/metallic CE =0/3"?
Original post by ambrin ox
Im really sorry but i didnt get that about the p orbitals? :/ pls help?

See the electrons fill up each orbital one at a time singly before they start pairing up as electrons are negatively charged and repel which is why the answer is 7 not 8 and in that sub shell the other 2 orbitals both contain 1 electron each
Original post by Philippamalko
It is in group 5, so has 5 outer electrons. so in AsH3 it would be bonded to 3 Hydrogen atoms leaving a lone pair (if you were drawing it)
i understand but 2 + 8 + 8 + 8 + 7 = 33 ?
could anyone tell me how to do 1b)iv) ?
http://store.aqa.org.uk/qual/gce/pdf/AQA-CHEM1-W-QP-JAN10.PDF
http://store.aqa.org.uk/qual/gce/pdf/AQA-CHEM1-W-QP-JAN10.PDF
Also, in hydrogen bonding, do both lone pairs on the Oxygen 'bond' with one of the hydrogens on another oxygen?
Original post by joker12345
could anyone tell me how to do 1b)iv) ?
http://store.aqa.org.uk/qual/gce/pdf/AQA-CHEM1-W-QP-JAN10.PDF
http://store.aqa.org.uk/qual/gce/pdf/AQA-CHEM1-W-QP-JAN10.PDF
There is no magic number, but it is using knowledge that once Mg has lost 2 electrons it now has a electronic configuration similar to that of a noble gas (full outer shell). The energy required to then remove the next electron is much greater.
Personally, I would've gone with around 7000 kJmol^-1.
Original post by It'sMeAgain
How do we know when to mention vdWs? For example, in Jan 11, Q5 (a) 'State the type of structure shown by a crystal of silicon.
Explain why the melting point of silicon is very high.'
I get that we say it is macromolecular with strong covalent bonds and need a lot of energy to break, but if we also say macromolecular so has lots of electrons and strong vdWs too, do we get 0 because mark scheme says "If IMF/H-bonds/Ionic/metallic CE =0/3"?
Explain why the melting point of silicon is very high.'
I get that we say it is macromolecular with strong covalent bonds and need a lot of energy to break, but if we also say macromolecular so has lots of electrons and strong vdWs too, do we get 0 because mark scheme says "If IMF/H-bonds/Ionic/metallic CE =0/3"?
You need to mention that it has a macromolecular structure. Many strong covalent bonds. These strong covalent bonds require lots of heat energy to break the bonds to result in melting.
The reason you couldn't mention vdW is because it doesn't have them, but also because it wouldn't actually be a contributing factor when compared with the covalent bonds.
You mention vdW for simple molecules, especially when comparing melting and boiling points of sulfur and phosphorus.
Original post by Tullia
You need to mention that it has a macromolecular structure. Many strong covalent bonds. These strong covalent bonds require lots of heat energy to break the bonds to result in melting.
The reason you couldn't mention vdW is because it doesn't have them, but also because it wouldn't actually be a contributing factor when compared with the covalent bonds.
You mention vdW for simple molecules, especially when comparing melting and boiling points of sulfur and phosphorus.
The reason you couldn't mention vdW is because it doesn't have them, but also because it wouldn't actually be a contributing factor when compared with the covalent bonds.
You mention vdW for simple molecules, especially when comparing melting and boiling points of sulfur and phosphorus.
Ok, thanks a lot =) It was really bothering and I was thinking I'd mess it up in the exam without even knowing it so I really appreciate the explanation and it finally makes sense. ^^ (Though I thought vdW were present in all molecules? Agh, I'm just going to accept it aha)
(edited 11 years ago)
Good luck everybody! 

Guys why is it foundation? what is the difference? I am not doing this board. I actually looked at the papers posted my the OP and was happy that I could do them, then saw that it was foundation. does this mean there is higher. if so then was grades can you get in them?
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- AQA A Level Economics Paper 1 (7136/1) - 15th May 2024 [Exam Chat]
- AQA GCSE Religious Studies A Paper 1 8062/17 - 16 May 2022 [Exam Chat]
- NO speaking exam date (and it's april!)
- GCSE Exam Discussions 2023
- AQA GCSE History Paper 1 (8145/1A/A-1B/E) - 15th May 2024 [Exam Chat]
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- Advice on applying after my gap year
- I failed my English Literature Coursework
- A Level Exam Discussions 2023
- Edexcel GCSE Religious Studies Paper 1 | 15th May 2023 [Exam Chat]
- Access to Centre Services
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- OCR GCSE Religious Studies Paper 1 [15 May 2023] Exam Chat
- As biology aqa paper 1
- School is killing me - Y11 "GYG" 2022
- study blog : self teaching : at 18 years old
- SQA Exam Discussions 2024
- Go ahead in time as far as possible
Latest
Trending
Last reply 1 hour ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 hour ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



