G482 help
Can someone explain this to me, seems quite simple but I don't understand it.
Question 2aii.
http://www.ocr.org.uk/Images/58130-question-paper-unit-g482-electrons-waves-and-photons.pdf
Question 2aii.
http://www.ocr.org.uk/Images/58130-question-paper-unit-g482-electrons-waves-and-photons.pdf
Original post by Super199
Can someone explain this to me, seems quite simple but I don't understand it.
Question 2aii.
http://www.ocr.org.uk/Images/58130-question-paper-unit-g482-electrons-waves-and-photons.pdf
Question 2aii.
http://www.ocr.org.uk/Images/58130-question-paper-unit-g482-electrons-waves-and-photons.pdf
It's where physics and chemistry overlap somewhat.
Batteries produce a reduction-oxidation (redox) chemical reaction to liberate electrons and create a charge imbalance between the electrodes. The reaction takes place between the electrode atoms in contact with an electrolyte solution.
Anions are atoms that have a net -ve charge, meaning the atom has gained additional electrons to the valence shell which now outnumber protons in the nucleus.
Cations are atoms that have a net +ve charge, meaning the atom has lost electrons from the valence shell.
Electrons are negatively charged and move freely through a conductor.
During discharge, Hydrogen (H+ cations) produced at the -ve plate move into the electrolyte which are transported via the electrolyte to the +ve plate.
HSO4- anions are consumed at both plates.
Original post by uberteknik
The question is asking you for the types of charge carrier moving within the stated medium.
It's where physics and chemistry overlap somewhat.
Batteries produce a reduction-oxidation (redox) chemical reaction to liberate electrons and create a charge imbalance between the electrodes. The reaction takes place between the electrode atoms in contact with an electrolyte solution.
Anions are atoms that have a net -ve charge, meaning the atom has gained additional electrons to the valence shell which now outnumber protons in the nucleus.
Cations are atoms that have a net +ve charge, meaning the atom has lost electrons from the valence shell.
Electrons are negatively charged and move freely through a conductor.
During discharge, Hydrogen (H+ cations) produced at the -ve plate move into the electrolyte which are transported via the electrolyte to the +ve plate.
HSO4- anions are consumed at both plates.
It's where physics and chemistry overlap somewhat.
Batteries produce a reduction-oxidation (redox) chemical reaction to liberate electrons and create a charge imbalance between the electrodes. The reaction takes place between the electrode atoms in contact with an electrolyte solution.
Anions are atoms that have a net -ve charge, meaning the atom has gained additional electrons to the valence shell which now outnumber protons in the nucleus.
Cations are atoms that have a net +ve charge, meaning the atom has lost electrons from the valence shell.
Electrons are negatively charged and move freely through a conductor.
During discharge, Hydrogen (H+ cations) produced at the -ve plate move into the electrolyte which are transported via the electrolyte to the +ve plate.
HSO4- anions are consumed at both plates.
Sorry I'm a tad confused. I thought the negative ions went to the positive terminal. How do the positive ions go the the positive terminal.
I don't understand why it is positive ions?

Original post by Super199
Sorry I'm a tad confused. I thought the negative ions went to the positive terminal. How do the positive ions go the the positive terminal.
I don't understand why it is positive ions?
I don't understand why it is positive ions?

Remember that in a conductor, the atoms are locked in position, it's only the electrons that are free to move.
In the electrolyte, cations (+ve atoms) are free to move because they are in aqueous solution and are not fixed in position.
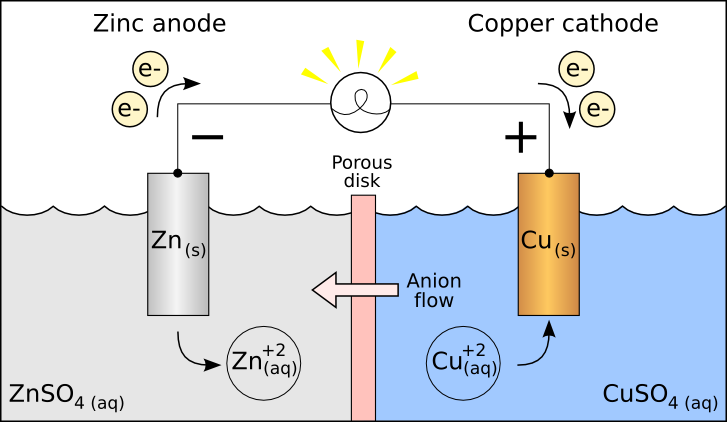
They say a picture is worth a thousand words.
These two diagrams shows the redox reactions clearly in two different battery compositions. Note the effective net flow of -ve charge is in a continuous loop.

Quick Reply
Related discussions
- Physics A G482 EWP Unofficial mark Scheme
- GCSE Exam Discussions 2024
- Unit 19 health and social care, HELP PLEASE!!
- Can anyone help me to do ROE For dental nursing please ?
- ways to study
- attachment issues
- Tips for time management
- Silver idea award
- Binomial Expansion Help
- Silver Idea Award/ Maker Resolution
- Punjabi GCSE (AQA) - Need urgent Help
- Unit 19: Study Skills Portfolio Building
- Period pain remedies?
- I need urgent help for my GSCEs
- how to set up uni halls room?
- PN1 Form
- your question's answered IDEA bronze and silver
- Passing second year/ is uni worth it
- Gcse english revision
- Finally been diagnosed with dyslexia - any advice?




