Specific Heat Capacity 1
So I’m new to this but I have a question on Specific Heat Capacity topic 1 in Physics. I’m taking GCSE’s if that helps.
The question: rearrange to make C the subject.
The equation is Change in Thermal Energy (J) = mass (Kg) x specific heat capacity (J/KgC) x change in temperature (C)
The question: rearrange to make C the subject.
The equation is Change in Thermal Energy (J) = mass (Kg) x specific heat capacity (J/KgC) x change in temperature (C)
Original post by ChrisMarcus
So I’m new to this but I have a question on Specific Heat Capacity topic 1 in Physics. I’m taking GCSE’s if that helps.
The question: rearrange to make C the subject.
The equation is Change in Thermal Energy (J) = mass (Kg) x specific heat capacity (J/KgC) x change in temperature (C)
The question: rearrange to make C the subject.
The equation is Change in Thermal Energy (J) = mass (Kg) x specific heat capacity (J/KgC) x change in temperature (C)
In algebraic notation, that equation can be written as Q = mcΔθ (where Q is the change in thermal energy, m is the mass, c is the specific heat capacity and Δθ is the temperature rise).
Because the right hand side of the equation is c times m times Δθ, what can you do to both sides of the equation so that the only thing on the right hand side is the c?
Spoiler
(edited 7 months ago)
If I get confused when re arranging an equation, I sometimes use an "equation triangle" to make it clearer for me. An example of one below:
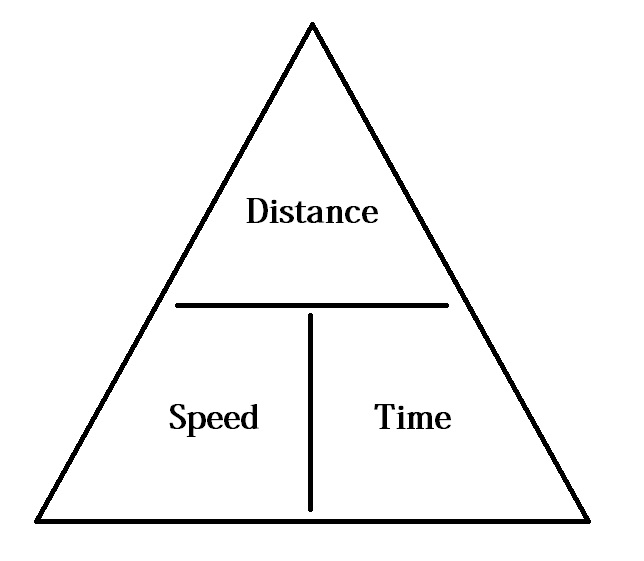
Of course, you are going to need to make one specifically for the specific heat capacity formula but one you are able to do this, it makes re arranging formulas much easier, considering you know how to use one afterwards. Feel free to reply to me and I could try to offer more help!

Of course, you are going to need to make one specifically for the specific heat capacity formula but one you are able to do this, it makes re arranging formulas much easier, considering you know how to use one afterwards. Feel free to reply to me and I could try to offer more help!
Quick Reply
Related discussions
- Thermal physics
- Physics problem-solving difficult thermodynamics/ latent heat/ heat capacity question
- Physics ice melting SHC and latent heat question
- A-level physics thermodynamics/ heat capacity/ latent heat difficult problem
- enthalpy change
- Biology maths question
- Chemistry enthalphy change question
- Enthalpy change what moles do I use?! Help!
- enthalpy change
- physics
- Required practicals
- calculate the total thermal energy
- Chemistry Specific Heat Capacity Question
- chem enthalpy change exam question
- Heat transfer between two objects
- A level thermal physics
- Physics A-level: Thermodynamics
- OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]
- Chemistry question
- in need of srs chem help - A-level




