how do you identify permanent dipole- permanent dipole molecules?
This makes zero sense.
I understand that CH4 is symmetrical and that CO2 is symmetrical also.
And I understand when there are two molecules like H2O so therefore it has permanent dipole- permanent dipole due to electro-negativities.
But how do you identify it when it says more than two elements (3 most of the time), for example CH3OH and CHCl3. I can't find any resources online what so ever regarding this. Not even Khan tells me what to do when there are three or more elements, nor does live-science tell me what to do (payed 80 pounds for it in subscription) and my teacher didn't mention it and it was set for homework.
What is it with dipoles today?
I understand that CH4 is symmetrical and that CO2 is symmetrical also.
And I understand when there are two molecules like H2O so therefore it has permanent dipole- permanent dipole due to electro-negativities.
But how do you identify it when it says more than two elements (3 most of the time), for example CH3OH and CHCl3. I can't find any resources online what so ever regarding this. Not even Khan tells me what to do when there are three or more elements, nor does live-science tell me what to do (payed 80 pounds for it in subscription) and my teacher didn't mention it and it was set for homework.
What is it with dipoles today?
Scroll to see replies
The forces are set up when there is a permenant dipole in the molecule.
Eg where there is a difference in electronegativity in a bond, for example C-O
Be careful though because if this is spread out evenly in the molcule the dipole cancels out.
Not sure if this makes sense, feel free to ask another question.
Eg where there is a difference in electronegativity in a bond, for example C-O
Be careful though because if this is spread out evenly in the molcule the dipole cancels out.
Not sure if this makes sense, feel free to ask another question.
You need to figure out the shape of the molecule and see whether the charge is more concentrated on one end of the molecule due to electronegativity, eg. CHCl3 should have permanent dipole-permanent dipole interactions because you have a delta positive carbon on one end, and three delta negative chlorine atoms on the other
Original post by Smelly Ellie
The forces are set up when there is a permenant dipole in the molecule.
Eg where there is a difference in electronegativity in a bond, for example C-O
Be careful though because if this is spread out evenly in the molcule the dipole cancels out.
Not sure if this makes sense, feel free to ask another question.
Eg where there is a difference in electronegativity in a bond, for example C-O
Be careful though because if this is spread out evenly in the molcule the dipole cancels out.
Not sure if this makes sense, feel free to ask another question.
Original post by Unkempt_One
You need to figure out the shape of the molecule and see whether the charge is more concentrated on one end of the molecule due to electronegativity, eg. CHCl3 should have permanent dipole-permanent dipole interactions because you have a delta positive carbon on one end, and three delta negative chlorine atoms on the other
Ok. So in CHCl3 we have:
C- a low electro-negativity
Cl - a higher electro negativity. Not to mention we have 3 of these.. so it's going to become even more powerful. So..
What happened to that hydrogen in the CHCl3? we didn't include it
compare electronegativites on the Pauling scale

Large difference = greater dipole charge

Large difference = greater dipole charge
Original post by ilyking
Ok. So in CHCl3 we have:
C- a low electro-negativity
Cl - a higher electro negativity. Not to mention we have 3 of these.. so it's going to become even more powerful. So..
What happened to that hydrogen in the CHCl3? we didn't include it
C- a low electro-negativity
Cl - a higher electro negativity. Not to mention we have 3 of these.. so it's going to become even more powerful. So..
What happened to that hydrogen in the CHCl3? we didn't include it
C and H have very similar electronegativity values, you just ignore it.
No dipole. CHCL3 the charges cancel as they are in all three 'arms' of the molecule. Overall no polarity.
Original post by Smelly Ellie
C and H have very similar electronegativity values, you just ignore it.
No dipole. CHCL3 the charges cancel as they are in all three 'arms' of the molecule. Overall no polarity.
No dipole. CHCL3 the charges cancel as they are in all three 'arms' of the molecule. Overall no polarity.
Not true, CHCl3 will have an overall dipole moment if you look at the structure in 3D
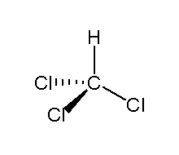
So there is a net negative charge on the "bottom" of the molecule and a net positive charge at the "top" of the molecule when seen as in the diagram
Original post by gingerbreadman85
compare electronegativites on the Pauling scale

Large difference = greater dipole charge

Large difference = greater dipole charge
Not what I'm looking for. I just don't understand when there is 3 elements involved.
2 elements is fine e.g. H2O, the oxygen is more electro negative so it is greedy over the electrons so H becomes slightly positive in relation to oxygen. Very very simple.
But what happens when there is three? > CHCl3, what happens now? does the C pull away electrons from H which also pulls electrons from Cl3? what the hell?
Original post by gingerbreadman85
Not true, CHCl3 will have an overall dipole moment if you look at the structure in 3D

So there is a net negative charge on the "bottom" of the molecule and a net positive charge at the "top" of the molecule when seen as in the diagram

So there is a net negative charge on the "bottom" of the molecule and a net positive charge at the "top" of the molecule when seen as in the diagram
mmm but can't you ignore the H? It has no effect. It only counts if there is a positive dipole at one end and negative at the other. I thought?
Original post by gingerbreadman85
Not true, CHCl3 will have an overall dipole moment if you look at the structure in 3D

So there is a net negative charge on the "bottom" of the molecule and a net positive charge at the "top" of the molecule when seen as in the diagram

So there is a net negative charge on the "bottom" of the molecule and a net positive charge at the "top" of the molecule when seen as in the diagram
Correct.
CCl4 has no overall dipole moment.
Original post by ilyking
Not what I'm looking for. I just don't understand when there is 3 elements involved.
2 elements is fine e.g. H2O, the oxygen is more electro negative so it is greedy over the electrons so H becomes slightly positive in relation to oxygen. Very very simple.
But what happens when there is three? > CHCl3, what happens now? does the C pull away electrons from H which also pulls electrons from Cl3? what the hell?
2 elements is fine e.g. H2O, the oxygen is more electro negative so it is greedy over the electrons so H becomes slightly positive in relation to oxygen. Very very simple.
But what happens when there is three? > CHCl3, what happens now? does the C pull away electrons from H which also pulls electrons from Cl3? what the hell?
Think about it individually
C-H, no net difference
C-Cl, Cl more electronegative
So EACH Cl will pull electron density away from the C, making it progressively more electropositive as more Cls are added around it. It is a cumulative effect, so CH3Cl will have a less electropositive carbon than CH2Cl2, and so on.
Look at the individual bonds, then add up the effects.
If for example one of the substituents was less electronegative than C, e.g. B, this would partially counteract the effect of the chlorine atoms.
Original post by Smelly Ellie
mmm but can't you ignore the H? It has no effect. It only counts if there is a positive dipole at one end and negative at the other. I thought?
The C-H bond itself has very little dipole character, but the molecule as a whole has quite a lot.
Sorry guys for the wrong information.
I was thinking CCl4
I was thinking CCl4

(edited 13 years ago)
Original post by Smelly Ellie
C and H have very similar electronegativity values, you just ignore it.
No dipole. CHCL3 the charges cancel as they are in all three 'arms' of the molecule. Overall no polarity.
No dipole. CHCL3 the charges cancel as they are in all three 'arms' of the molecule. Overall no polarity.
What? They are in three arms of the molecule indeed, but those three arms form a tripod in 3D space so there must be a dipole.
Original post by Unkempt_One
What? They are in three arms of the molecule indeed, but those three arms form a tripod in 3D space so there must be a dipole.
JEEZ I already said I was mistaken leave me be

Original post by Smelly Ellie
Sorry guys for the wrong information.
I was thinking CCl4
I was thinking CCl4

Yeah, that has no dipole.
Original post by Smelly Ellie
JEEZ I already said I was mistaken leave me be 

Sorry, I joined the thread before all of those people correcting you.

Original post by Unkempt_One
Sorry, I joined the thread before all of those people correcting you. 

Haha what I originally said was kinda right. Then i got confuddled. Man I neeeed an A* in chemi as well haha. Silly billy me.
a molecule with dipole(s) needs a center of symmetry not to have an overall dipole moment.
Center of symmetry
A molecule has a center of symmetry when, for any atom in the molecule, an identical atom exists diametrically opposite this center an equal distance from it. There may or may not be an atom at the center. Examples are xenon tetrafluoride (XeF4) where the inversion center is at the Xe atom, and benzene (C6H6) where the inversion center is at the center of the ring.
Center of symmetry
A molecule has a center of symmetry when, for any atom in the molecule, an identical atom exists diametrically opposite this center an equal distance from it. There may or may not be an atom at the center. Examples are xenon tetrafluoride (XeF4) where the inversion center is at the Xe atom, and benzene (C6H6) where the inversion center is at the center of the ring.
Quick Reply
Related discussions
- AQA A level chemistry
- Chemistry question dipole
- Help recognising intermolecular forces
- Using your knowledge of chemistry
- is permant dipole dipole forces same as a permanent dipole?
- dipole dipole vs vanderwaals forces difference
- would you expect cl2 to conduct electricty
- how do u know when to say ions/electrons
- a level chemistry
- van der waals
- Boiling point of halogenoalkanes
- Chemistry question dipole
- Ask Us Anything!
- are there more polar or nonpolar molecules?
- biology
- multiple choice Q
- BSF polarity
- A Level Chemistry help
- Learndirect- presentation advice
- Hi chem a level polar bonds help
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products


