Mass Spectrometry question
Question: Look at figure 29.41(see below) which shows the mass spectrum of ethanol, C2H5OH. A structural isomer of ethanol is methoxymethane, an ether with the formula CH3OCH3.
a) Predict the mass-to-charge ratio of a fragment that would appear on the mass spectrum of methoxymethane but does not appear on ethanol's mass spectrum.
b) Give the formula of ion responsible for the peak in your answer to part a.
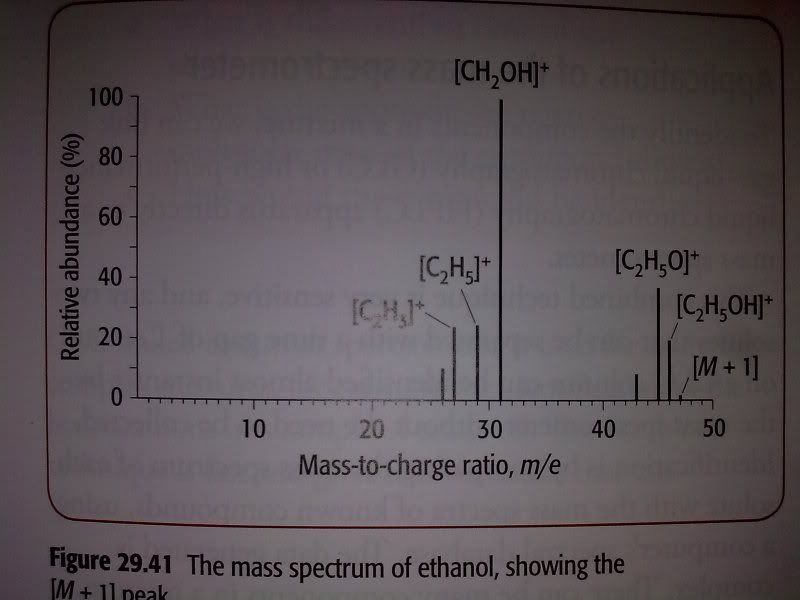
I think that answer to this question is wrong in the book. The figure doesn't show a peak at 15 m/e(It actually should!!), so the answer to a should be 15 and the ion responsible for it should be CH3+ - whereas book gives 31 and CH3O+ as the answer. I think book is wrong because there's also a peak at 31 m/e on mass spectrum of ethanol . . . Just wanted to ask if I'm wrong?!
a) Predict the mass-to-charge ratio of a fragment that would appear on the mass spectrum of methoxymethane but does not appear on ethanol's mass spectrum.
b) Give the formula of ion responsible for the peak in your answer to part a.

I think that answer to this question is wrong in the book. The figure doesn't show a peak at 15 m/e(It actually should!!), so the answer to a should be 15 and the ion responsible for it should be CH3+ - whereas book gives 31 and CH3O+ as the answer. I think book is wrong because there's also a peak at 31 m/e on mass spectrum of ethanol . . . Just wanted to ask if I'm wrong?!

Are you saying there should be a peak for 15 for ethanol? The 15 peak is more likely to be seen in methoxymethane and that seems like a more sensible answer.
The book contradicts itself, so that can't be trusted
The book contradicts itself, so that can't be trusted

Original post by EierVonSatan
Are you saying there should be a peak for 15 for ethanol? The 15 peak is more likely to be seen in methoxymethane and that seems like a more sensible answer.
The book contradicts itself, so that can't be trusted
The book contradicts itself, so that can't be trusted

Yeah, but the figure in the book doesn't show that peak (maybe it's because of low resolution)
 .
.Just another point - it may be because of the fact that I'm misreading the question! Does the book mean that which fragment's peak appear on the spectrum of methoxymethane, but not on that of ethanol? It may be just another point that charge to mass of ratios of [CH3O]+ and [CH2OH]+ are same. . .


I don't think you're interpreting the question wrong, it's looking for a peak that would be on methoxymethane's spectrum but not on ethanol's.
I'm unfamiliar of how much you're expected to cover on this topic. As far as I can see the question is nonsense and I'd move on if I were you

Original post by EierVonSatan
I don't think you're interpreting the question wrong, it's looking for a peak that would be on methoxymethane's spectrum but not on ethanol's.
I'm unfamiliar of how much you're expected to cover on this topic. As far as I can see the question is nonsense and I'd move on if I were you
I'm unfamiliar of how much you're expected to cover on this topic. As far as I can see the question is nonsense and I'd move on if I were you

 Many thanks!
Many thanks! 
Hello again, I've got another question. 
About the mass spectrum of molecule containing two bromine atoms, the book says, "The M, [M +2] and [M + 4] peaks also occur in dibromomethane but the relative heights are easier to work out. Because the ratio 79Br:81Br is 1 : 1, we get the M : [M + 2] : [M + 4] height ratio as 1 : 2 : 1"
My question is that shouldn't the M : [M + 2] : [M + 4] height ratio be 1 : 1 : 1? Or am I missing a bit of maths?
-Thanks

About the mass spectrum of molecule containing two bromine atoms, the book says, "The M, [M +2] and [M + 4] peaks also occur in dibromomethane but the relative heights are easier to work out. Because the ratio 79Br:81Br is 1 : 1, we get the M : [M + 2] : [M + 4] height ratio as 1 : 2 : 1"
My question is that shouldn't the M : [M + 2] : [M + 4] height ratio be 1 : 1 : 1? Or am I missing a bit of maths?

-Thanks
Original post by Zishi
Hello again, I've got another question. 
About the mass spectrum of molecule containing two bromine atoms, the book says, "The M, [M +2] and [M + 4] peaks also occur in dibromomethane but the relative heights are easier to work out. Because the ratio 79Br:81Br is 1 : 1, we get the M : [M + 2] : [M + 4] height ratio as 1 : 2 : 1"
My question is that shouldn't the M : [M + 2] : [M + 4] height ratio be 1 : 1 : 1? Or am I missing a bit of maths?
-Thanks

About the mass spectrum of molecule containing two bromine atoms, the book says, "The M, [M +2] and [M + 4] peaks also occur in dibromomethane but the relative heights are easier to work out. Because the ratio 79Br:81Br is 1 : 1, we get the M : [M + 2] : [M + 4] height ratio as 1 : 2 : 1"
My question is that shouldn't the M : [M + 2] : [M + 4] height ratio be 1 : 1 : 1? Or am I missing a bit of maths?

-Thanks
List the combinations:
79 + 79 = M
79 + 81 = M + 2
81 + 79 = M + 2
81 + 81 = M + 4
so there would be a 1 : 2 : 1 ratio (it will follow pascals triangle
 )
)Original post by EierVonSatan
List the combinations:
79 + 79 = M
79 + 81 = M + 2
81 + 79 = M + 2
81 + 81 = M + 4
so there would be a 1 : 2 : 1 ratio (it will follow pascals triangle )
)
79 + 79 = M
79 + 81 = M + 2
81 + 79 = M + 2
81 + 81 = M + 4
so there would be a 1 : 2 : 1 ratio (it will follow pascals triangle
 )
)Wow, things are rarely as simple as they seem to be! Many thanks.

Hello! The answer is right in the book as the question is asking for a fragment that is possible in methoxymthane but not in ethanol. Remember that different fragments can have the same mass. If we compare the structure of ethanol and methoxymethane then we will find that CH3O+ fragment is only possible in methoxymethane structure.
Original post by 123alpha
Hello! The answer is right in the book as the question is asking for a fragment that is possible in methoxymthane but not in ethanol. Remember that different fragments can have the same mass. If we compare the structure of ethanol and methoxymethane then we will find that CH3O+ fragment is only possible in methoxymethane structure.
The question is asking for a m/z ratio that exists in methoxymethane but not ethanol - not what the identity of that fragment is. The CH3O+ fragment would have a ratio of 31, which is clearly on the spectrum of ethanol posted above (as CH2OH+).
CH3+ would be m.z of 15, which is possible from methoxymethane but is not present in ethanols MS. Here is the mass spectrum of methoxymethane showing that peak at 15.
I need help I am looking at the mass spectrum for luminol and I am tying to name and Draw the structure of the fragments which corresponds to the peak at 119 m/z, 92 m/z and 91 m/zI have put the mass spectrum below if anyone can help me it would be gladly appreciated thanks  https://webbook.nist.gov/cgi/cbook.cgi?ID=C521313&Mask=200#Mass-Spec
https://webbook.nist.gov/cgi/cbook.cgi?ID=C521313&Mask=200#Mass-Spec
 https://webbook.nist.gov/cgi/cbook.cgi?ID=C521313&Mask=200#Mass-Spec
https://webbook.nist.gov/cgi/cbook.cgi?ID=C521313&Mask=200#Mass-SpecQuick Reply
Related discussions
- a level chemistry
- Any Chemistry Mass Spectrum nerds help !!!!!!
- Mass spectrometry
- chemistry help please!!!
- Mass spectrometry
- OCR A Level Chemistry
- Chemistry A level,mass spectrometry questions
- mass spectrometry and rearranging the kinetic energy equation
- A level chemistry time of flight mass spectrometry questions
- A2 chemistry question relating to mass spectrometry.
- Chemistry mass spectrometry .HELP PLS !!!
- surviving (hopefully) sixth form | year 12 journal
- Biochemistry Personal Statement Example
- Enumeration of thiol groups in ovalbumin
- PAG 1.2 chemistry A-Level OCR A
- Binding Energy (Isaac Physics J4.8 Part A)
- Physics mass-energy question.
- Chemistry a level enthalpy
- How to know which number in the ratio is to one? (Please help)
- Hydrated Salts question
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



