A nucleophile by definition is a species with a lone pair of electrons that is used to form a covalent bond with a δ+ atom in another molecule. NH3 has a lone pair. 

Original post by thymolphthalein
A nucleophile by definition is a species with a lone pair of electrons that is used to form a covalent bond with a δ+ atom in another molecule. NH3 has a lone pair. 

Original post by urkadee
Also note if there had been an NH2 in the options, the answer would have been NH2, as it has 2 lone pairs of electrons, so it would be more reactive as a nucleophile 

thank you

but doesnt bromine have three lone pairs around each atom? surely that makes it more nucleophillic?
thnx again
I need sleep- I read that as "how do you identify as a nucleophile?" 😂
Original post by ah4p
thank you 
but doesnt bromine have three lone pairs around each atom? surely that makes it more nucleophillic?
thnx again

but doesnt bromine have three lone pairs around each atom? surely that makes it more nucleophillic?
thnx again
I guess I wasn't clear enough earlier, sorry.

Nucleophiles have to be negatively charged ions OR have a strong δ- charge on the molecule (for example, due to the lone pair on NH3).
So although the bromide ion can be a nucleophile, bromine (Br2) which is given as an option would be incorrect as it is a neutral molecule. It has no significant polarity.

If I'm being too weirdly unclear about this:
http://www.chemguide.co.uk/mechanisms/nucsub/whatis.html
Chemguide is a pretty neat website.

Original post by thymolphthalein
I guess I wasn't clear enough earlier, sorry.
Nucleophiles have to be negatively charged ions OR have a strong δ- charge on the molecule (for example, due to the lone pair on NH3).
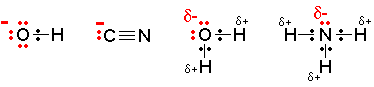
So although the bromide ion can be a nucleophile, bromine (Br2) which is given as an option would be incorrect as it is a neutral molecule. It has no significant polarity.
If I'm being too weirdly unclear about this:
http://www.chemguide.co.uk/mechanisms/nucsub/whatis.html
Chemguide is a pretty neat website.

Nucleophiles have to be negatively charged ions OR have a strong δ- charge on the molecule (for example, due to the lone pair on NH3).
So although the bromide ion can be a nucleophile, bromine (Br2) which is given as an option would be incorrect as it is a neutral molecule. It has no significant polarity.

If I'm being too weirdly unclear about this:
http://www.chemguide.co.uk/mechanisms/nucsub/whatis.html
Chemguide is a pretty neat website.

ah ok thank you v v much
 that makes complete sense now
that makes complete sense nowQuick Reply
Related discussions
- AS chemistry paper 2 2022 AQ
- SN1 and SN2 reactions (chemistry)
- Electrophillic/nucelophillic substitution /addition
- Could someone please help with a mechansim question.
- Chemistry help🙏🏼🙏🏼
- Nucelophiles Vs Ligands
- Ask Us Anything!
- OCR AS Chemistry- nucleophilic subs. reaction
- Do you need to know Acylation mechanism for A level OCR chemistry ?
- Organic chemistry learning a-level
- A level Chemistry Uplearn
- Random OCR A-level organic mechanism reactions paper 3
- Organuc chemistry A level question
- A level chemistry
- Organic Synthesis help
- A-level Chemistry: Mechanisms
- amines
- Reaction Mechanism of Allyl Bromide and (aqueous) NaOH
- AQA A-Level Chemistry Paper 2 (7405/2) - 19th June 2023 [Exam Chat]
- Why does Nitrogen gain a positive charge in this mechanism ?
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products




