Cambridge Physics Problem 20: Thermal System Problem
Two cylinders A and C of equal volume V contain the same ideal gas at temperature T and at pressures 2p and p respectively. A valve connecting the two cylinders is opened slightly and as the gas leaks from A to B, the pressure in A is maintained at 2p by pushing in a piston. The process is continued until the gas in cylinder B is also at 2p. If there is good thermal contact between the cylinders but the are thermally insulated from their surroundings, find:
(i) The final temperature in terms of T
(ii) the final volume of gas in cylinder A in terms of V.
Before I attempt the question, may I ask if the final temperature will be the same for both sides of the cylinder? What about the pressure? I'm still visualising the problem. What other tips can you give me? Thank you!
(i) The final temperature in terms of T
(ii) the final volume of gas in cylinder A in terms of V.
Before I attempt the question, may I ask if the final temperature will be the same for both sides of the cylinder? What about the pressure? I'm still visualising the problem. What other tips can you give me? Thank you!
Yes good "thermal contact" should mean that the cylinders reach thermal equilibrium rapidly, the pressure in A is kept constant while the pressure in B increases as the moles of gas there increases.
Tips: this is kinda dependent on my answer being right but it seems reasonable :P , anyway...
Tips: this is kinda dependent on my answer being right but it seems reasonable :P , anyway...
Spoiler
Any other comments from anyone?
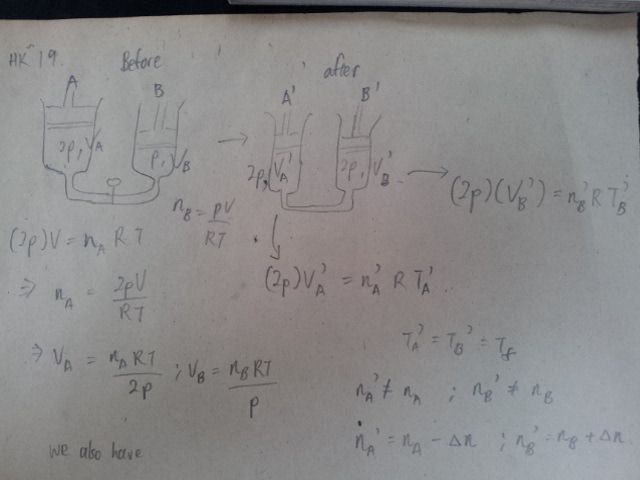
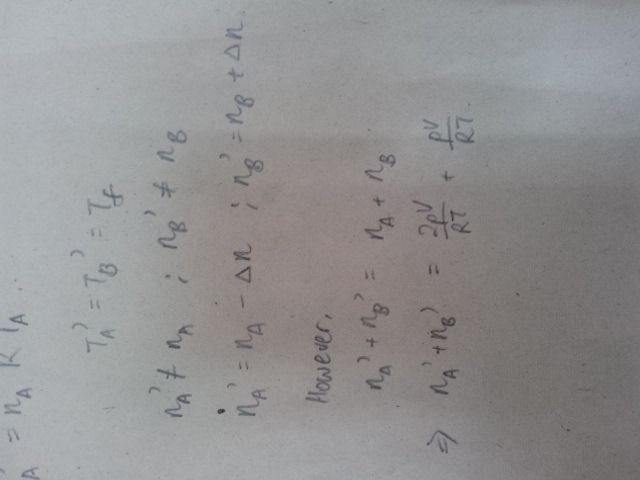
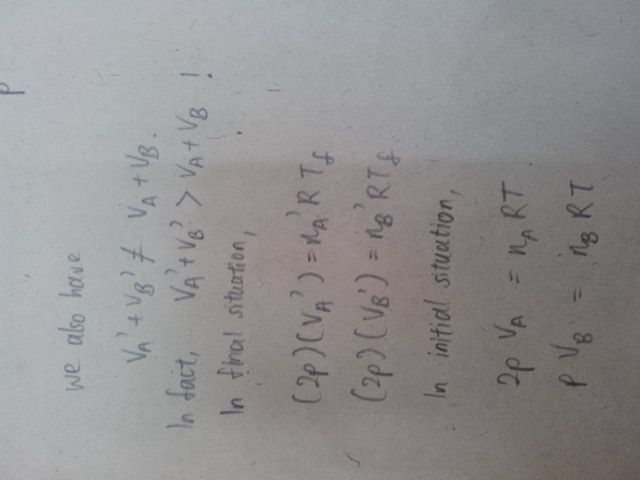
I'm having trouble eliminating the VA' and VB' . Can anyone help me on this?
Is molar heat capacity necessary to solve the question?
Quick Reply
Related discussions
- Can I get a Post-Graduate Physics degree after PPE?
- Aeronautical & Aerospace Engineering
- How To Get An Oxford Engineering Offer: Chapter 3 - Super-curricular and Resources
- Alevel physics in engineering
- supercurriculars for engineering + general tips and advice?
- Want to switch course
- Low AS UMS, should I give up on propspects of Oxbridge?
- Cambridge maths Vs CS .
- Can you take both Cambridge and edxcel
- Shoot for the Stars 2- Crash course into uni Maths&Physics
- Architecture and structural engineering
- Third A level for Mathematics course: CS vs Psychology
- Cambridge Natsci Admissions
- I don't know what subject to pick
- i am really confused
- Should I choose Aerospace or Electrical/Electronics ?
- Computer Science or Economics for investment banking
- I got 9 A*'s in my GCSE's - Ask Me Anything
- Use a simulator to plot the frequency response of the circuit
- Cambridge Demystified




