This discussion is now closed.
Check out other Related discussions
- Why does Scandium only exist in the 3+ ion
- Atomic Structure - Shapes of S and P orbitals AQA a level chem
- Chem a level
- CHEM stupid singly orbitals
- chemistry question
- Lewis structure for SO4 2-
- Alevel physics
- Relative atomic mass
- Bond breaking and intermolecular force A level
- NF3 lewis structure
- Describe how sodium conducts thermal energy. (3 marks) GCSE AQA
- SPecific charge - physics homework
- Magnesium melting point
- chemistry multiple choice
- AQA A-Level Chemistry
- Random OCR A-level organic mechanism reactions paper 3
- Chemistry Questions
- orbitals and subshells
- shape of molecules a level chem
- Chemistry A-level exam q help!
Electronic structure of atoms (sub levels).
Hi,
when drawing the simple electron structures of elements such as sodium:
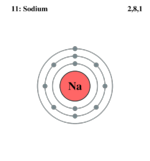
I understand that the electrons are filled in the shells such that the first shell is filled with 2 electrons, the second 8 and the third 8 (or in the case of sodium just 1). However what is confusing me is that when we write out the full electronic structures with the principle energy levels (1,2,3,4) and the sub levels (s,p,d,f), in the first principal energy level there are 2 electrons, then the second there are 8 electrons (which follows the trend I understand) then for the third principal energy level it says it can contain a maximum number of 18 electrons and the forth can contain 32. I have always understood that that the third shell could contain only 8 electrons and i though it was 8 for the fourth shell also.
I have always understood that that the third shell could contain only 8 electrons and i though it was 8 for the fourth shell also.
very grateful if someone can explain this.
cheers.
when drawing the simple electron structures of elements such as sodium:

I understand that the electrons are filled in the shells such that the first shell is filled with 2 electrons, the second 8 and the third 8 (or in the case of sodium just 1). However what is confusing me is that when we write out the full electronic structures with the principle energy levels (1,2,3,4) and the sub levels (s,p,d,f), in the first principal energy level there are 2 electrons, then the second there are 8 electrons (which follows the trend I understand) then for the third principal energy level it says it can contain a maximum number of 18 electrons and the forth can contain 32.
 I have always understood that that the third shell could contain only 8 electrons and i though it was 8 for the fourth shell also.
I have always understood that that the third shell could contain only 8 electrons and i though it was 8 for the fourth shell also.very grateful if someone can explain this.
cheers.
Sorry if I'm stating the obvious here, but if you count along the periodic table horizotally from Lithium to Berilium, there are two- meaning 2 electrons in the 's' level. Count along from Sc to Zn and there are 10 elements- meaning 10 in the 'd' block. Count from B to Ne and there are 6- meaning a six electron capacity in the 'p' block.
Again, sorry if I'm stating the obvious here. In the first shell, we only have 2 electrons, which is just the 's' level. Onto the second shell- we have one 's'- 2 electrons. There are 6 electrons left over, so we have 6 in the 'd' block. Next is the same, as it holds only 8 electrons as well.
They're just a couple of basics.
Again, sorry if I'm stating the obvious here. In the first shell, we only have 2 electrons, which is just the 's' level. Onto the second shell- we have one 's'- 2 electrons. There are 6 electrons left over, so we have 6 in the 'd' block. Next is the same, as it holds only 8 electrons as well.
They're just a couple of basics.
I know it sounds really confusing. The sub levels are kind of the shape of the shell that the electron is in. Have a look at this wikipedia page: http://en.wikipedia.org/wiki/Electron_configuration
It might help a bit, and i'm sorry if it isn't that much help. Its been quite a few months since i did my a levels, then again, i never did question why it went 1s2 2s2 2p6 3s2 3p6 4s2 3d1, i mean it would imply that there are 9 electrons in the 3rd shell.
It might help a bit, and i'm sorry if it isn't that much help. Its been quite a few months since i did my a levels, then again, i never did question why it went 1s2 2s2 2p6 3s2 3p6 4s2 3d1, i mean it would imply that there are 9 electrons in the 3rd shell.
Barry Chuckle
very grateful if someone can explain this.
At GCSE you were taught quite a significant simplification, that the order of filling is 2, 8, 8 and so you were never asked to give electronic configurations for anything heavier than calcium.
The 'real' ordering is more complicated than this - 2, 8, 18, 18, 32, 32
Related discussions
- Why does Scandium only exist in the 3+ ion
- Atomic Structure - Shapes of S and P orbitals AQA a level chem
- Chem a level
- CHEM stupid singly orbitals
- chemistry question
- Lewis structure for SO4 2-
- Alevel physics
- Relative atomic mass
- Bond breaking and intermolecular force A level
- NF3 lewis structure
- Describe how sodium conducts thermal energy. (3 marks) GCSE AQA
- SPecific charge - physics homework
- Magnesium melting point
- chemistry multiple choice
- AQA A-Level Chemistry
- Random OCR A-level organic mechanism reactions paper 3
- Chemistry Questions
- orbitals and subshells
- shape of molecules a level chem
- Chemistry A-level exam q help!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products


