Edexcel chemistry - unit 2 19th january 2012
Scroll to see replies
Original post by Cetacea
What would you observe if you added potassium iodide to conc. sulphuric acid?
Purple vapour?
Original post by cisne
Purple vapour?
That's correct. There are 2 other main observations though - a smell of bad eggs (from the H2S gas) and a yellow solid may form.
Next question: Explain why boron trichloride, BCl3, is non-polar despite having polar bonds.
(edited 12 years ago)
Original post by Cetacea
What would you observe if you added potassium iodide to conc. sulphuric acid?
smell of rotten eggs (hydrogen sulphide), purple vapour from the iodine and solid yellow sulphur?
Original post by cisne
but-2-ene exists as two geometrical isomers, explain why but-2-ene exists as two geometrical isomers. (2 marks)
this is a question from january 2008,though......i cuouldn't find a simple way to answer this,can anybody help me please?
this is a question from january 2008,though......i cuouldn't find a simple way to answer this,can anybody help me please?
because you can have e-z isomerism?
for example
remember there is a c=c double bond, your priority group (CH3) can be either on the same side of the c=c bond or can be on the opposite side .
Original post by Cetacea
That's correct. There are 2 other main observations though - a smell of bad eggs (from the H2S gas) and a yellow solid may form.
Next question: Explain why boron trichloride, BCl3, is non-polar despite having polar bonds.
Next question: Explain why boron trichloride, BCl3, is non-polar despite having polar bonds.
What's the full equation for the reaction between sulphuric acid and potassium iodide then?
For the boron trichloride: All ends of the compound from top to bottom, and left to right are slightly negative. So it only relies on dispersion forces?
Original post by James A
because you can have e-z isomerism?
for example
remember there is a c=c double bond, your priority group (CH3) can be either on the same side of the c=c bond or can be on the opposite side .
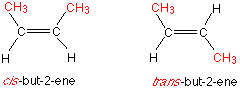
for example
remember there is a c=c double bond, your priority group (CH3) can be either on the same side of the c=c bond or can be on the opposite side .
yeah....thanks alot...
Original post by cisne
this is something i prepared with the help of my revision guide....my revision guide is really helpful..i'll try to upload more..
THANK YOUUU SO MUCH!!
They really helpful

Original post by Cetacea
That's correct. There are 2 other main observations though - a smell of bad eggs (from the H2S gas) and a yellow solid may form.
Next question: Explain why boron trichloride, BCl3, is non-polar despite having polar bonds.
Next question: Explain why boron trichloride, BCl3, is non-polar despite having polar bonds.
because the dipoles moments cancel each other out
Helllooo 
I'm going through the alcohols topic yeh.. & i came across 2 contracting stuff..
first of all..would the reaction be affected if we use alcoholic ammonia & just excess ammonia..??
According to the AS chem blue text book it says
CH3CH2I + NH3 ---> CH3CH2NH2 + HI
Where as in the george facer textbook,
When excess ammonia is used, it forms
CH3CH2CH2Cl + 2NH3 ---> CH3CH2CH2NH2 + NH4Cl
& it even says stating HCl as a product of this reaction is a common error in AS ???
Which one is correct???

I'm going through the alcohols topic yeh.. & i came across 2 contracting stuff..
first of all..would the reaction be affected if we use alcoholic ammonia & just excess ammonia..??
According to the AS chem blue text book it says
CH3CH2I + NH3 ---> CH3CH2NH2 + HI
Where as in the george facer textbook,
When excess ammonia is used, it forms
CH3CH2CH2Cl + 2NH3 ---> CH3CH2CH2NH2 + NH4Cl
& it even says stating HCl as a product of this reaction is a common error in AS ???

Which one is correct???

Original post by aqua05
Original post by aqua05
Helllooo 
I'm going through the alcohols topic yeh.. & i came across 2 contracting stuff..
first of all..would the reaction be affected if we use alcoholic ammonia & just excess ammonia..??
According to the AS chem blue text book it says
CH3CH2I + NH3 ---> CH3CH2NH2 + HI
Where as in the george facer textbook,
When excess ammonia is used, it forms
CH3CH2CH2Cl + 2NH3 ---> CH3CH2CH2NH2 + NH4Cl
& it even says stating HCl as a product of this reaction is a common error in AS ???
Which one is correct???

I'm going through the alcohols topic yeh.. & i came across 2 contracting stuff..
first of all..would the reaction be affected if we use alcoholic ammonia & just excess ammonia..??
According to the AS chem blue text book it says
CH3CH2I + NH3 ---> CH3CH2NH2 + HI
Where as in the george facer textbook,
When excess ammonia is used, it forms
CH3CH2CH2Cl + 2NH3 ---> CH3CH2CH2NH2 + NH4Cl
& it even says stating HCl as a product of this reaction is a common error in AS ???

Which one is correct???

Both are technically correct but the HI in the first one would go onto react with another NH3 molecule to form NH4I. The overall equation would be the second one.
Original post by NutterFrutter
Both are technically correct but the HI in the first one would go onto react with another NH3 molecule to form NH4I. The overall equation would be the second one.
righhht

Makes sense !
Basically in the exam we should write the second equation, isnt it ?!
Thanksss !

Original post by aqua05
Original post by aqua05
righhht 
Makes sense !
Basically in the exam we should write the second equation, isnt it ?!
Thanksss !

Makes sense !
Basically in the exam we should write the second equation, isnt it ?!
Thanksss !

Yeah, they'd most likely expect the overall equation in the exam.

Original post by NutterFrutter
Both are technically correct but the HI in the first one would go onto react with another NH3 molecule to form NH4I. The overall equation would be the second one.
btw what resources are you using to study ?
Original post by aqua05
Original post by aqua05
btw what resources are you using to study ?
CGP revision guide and the Edexcel textbook.
Original post by James A
smell of rotten eggs (hydrogen sulphide), purple vapour from the iodine and solid yellow sulphur?
Original post by James A
because the dipoles moments cancel each other out
Both correct!
Original post by Cetacea
Both correct!
woop woop, we need to fire more questions and make it a big Q&A so we can all revise together
Original post by James A
woop woop, we need to fire more questions and make it a big Q&A so we can all revise together
Name all of the intermolecular forces operating in molecules of propanal.
Original post by Cetacea
Name all of the intermolecular forces operating in molecules of propanal.
so propanal is an aldehyde, therefore NO HYDROGEN BONDS OCCUR, however PERMANENT DIPOLE DIPOLE interactions OCCUR and of course LONDON FORCES OCCURS IN ALL MOLECULES MOLECULES
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- Edexcel chemistry unit 2 mixed questions
- GCSE Exam Discussions 2023
- AS chemistry past paper help
- Official thread - January 2023 IAL edexcel
- IAL repeats cash in.
- BTEC- Business Level 3 Revision help
- A Level Advice
- unit 3 applied science Jan 2024
- BTEC Applied Science Exam Revision
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- Is it possible to change sixth forms now (in year 12)?
- iria's 'grow your grades' blog :)
- BTEC Engineering Level 3 Unit 6
- Synoptic questions
- Edexcel GCSE Computer Science Paper 1 (1CP2 01) - 19th May 2023 [Exam Chat]
- Btec applied science unit 3 2024 exam
- Edexcel Past Papers
Latest
Trending
Last reply 1 day ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 3 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 3 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 day ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 3 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 3 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 4 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



