This discussion is now closed.
Check out other Related discussions
- BMAT Practice Papers
- UCAT Medentry QR Question
- Do I need to do the BMAT to get an interview ?
- Low Ucat advice for med uni
- Bmat cut off
- BMAT - Will a low BMAT score affect your application to non-BMAT universities?
- BMAT Isc medical 700 questions book
- BMAT plz help me
- BMAT Medicine 2024
- is it still worth taking the BMAT
- Oxford medicine ?
- Medicine with 2:2
- Problems accessing my BMAT results
- BMAT 2023 help
- Medicine entry 2024
- Will I be taking the BMAT?
- How should I plan doing both UCAT & BMAT?
- Allied Healthcare Careers with Potential job prospects in UAE
- Bmat scoring
- Is it over?
*MEGATHREAD* - BMAT for 2016 Entry Discussion
Scroll to see replies
Original post by ilovecake123
please can someone help with this question
The way i worked out this question was by firstly, working out the area of the largest dark circle, and then subtracting the area of the largest white circle. This would give the area of the larger shaded dark section. Than i did the same with the 2 smaller circles. This would give you area of the smaller shaded dark section. Then add them together and theres your answer!
Hope that helps x
Posted from TSR Mobile
Anyone willing to mark a section 3 essay?  I also don't mind marking
I also don't mind marking
 I also don't mind marking
I also don't mind markingi need help with all these answers hope someone can explain them to me! thanks
Original post by ilovecake123
please can someone explain these pls?
Alpha is made of two protons and two neutrons, therefore it is poor at penetrating through materials. Therefore when you put a piece of, for example, alluminium, infront of it, the detector would not detect it!
Beta is fairly good at penetration and gamma is very good. So i came to the last answer, because even when the sheets of material are placed infront of the radiation beam, they are still detected
Posted from TSR Mobile
Original post by Rp165
The way i worked out this question was by firstly, working out the area of the largest dark circle, and then subtracting the area of the largest white circle. This would give the area of the larger shaded dark section. Than i did the same with the 2 smaller circles. This would give you area of the smaller shaded dark section. Then add them together and theres your answer!
Hope that helps x
Posted from TSR Mobile
Hope that helps x
Posted from TSR Mobile
thanks
Original post by Rp165
Alpha is made of two protons and two neutrons, therefore it is poor at penetrating through materials. Therefore when you put a piece of, for example, alluminium, infront of it, the detector would not detect it!
Beta is fairly good at penetration and gamma is very good. So i came to the last answer, because even when the sheets of material are placed infront of the radiation beam, they are still detected
Posted from TSR Mobile
Beta is fairly good at penetration and gamma is very good. So i came to the last answer, because even when the sheets of material are placed infront of the radiation beam, they are still detected
Posted from TSR Mobile
thanks
can you do any of the others, thanks so much!
Original post by ilovecake123
please can someone explain these answers
This is related to the reactivity series. So you got to think that anything that is less reactive than vanadium and zinc cannot be used to obtain the metal from its ore
Posted from TSR Mobile
Original post by Rp165
This is related to the reactivity series. So you got to think that anything that is less reactive than vanadium and zinc cannot be used to obtain the metal from its ore
Posted from TSR Mobile
Posted from TSR Mobile
ahh ok thanks!
sorry i dont get why i can't be b or d or e
D is the correct answer?
pls could you explain it
(edited 8 years ago)
Original post by aspiring_
Anyone willing to mark a section 3 essay?  I also don't mind marking
I also don't mind marking
 I also don't mind marking
I also don't mind markingI wouldn't mind

Is anyone else just loosing motivation to revise for the BMAT? Like, I'm just sitting here and should be doing questions but just don't have the will to...
All of the metals mentioned in the answers are more reactive than Vanadium so they will displace the sulfate from it to form a metal sulfate and vanadium. Only iron is less reactive than vanadium so it cannot displace the sulfate from the vanadium,
Hope this helps!
Hope this helps!
Original post by ilovecake123
ahh ok thanks!
sorry i dont get why i can't be b or d or e
D is the correct answer?
pls could you explain it
sorry i dont get why i can't be b or d or e
D is the correct answer?
pls could you explain it
Yeah, especially section 1 when I'm 10 questions in and can't be bothered any more!
Original post by cookiemonster15
Is anyone else just loosing motivation to revise for the BMAT? Like, I'm just sitting here and should be doing questions but just don't have the will to...
Original post by santh1234
All of the metals mentioned in the answers are more reactive than Vanadium so they will displace the sulfate from it to form a metal sulfate and vanadium. Only iron is less reactive than vanadium so it cannot displace the sulfate from the vanadium,
Hope this helps!
Hope this helps!
AHHH thanks!
any idea where you got this info from cause i wanna do some more reading on this?
Original post by Hopefulmedic15
90 students had A-C grades in their mock and in their real exams and 60 students had other grades in the mock and real exams so 90+60=150
150/total = 150/(150+25+25) = 150/200
= 75%
Posted from TSR Mobile
150/total = 150/(150+25+25) = 150/200
= 75%
Posted from TSR Mobile
Thank you!

Original post by santh1234
All of the metals mentioned in the answers are more reactive than Vanadium so they will displace the sulfate from it to form a metal sulfate and vanadium. Only iron is less reactive than vanadium so it cannot displace the sulfate from the vanadium,
Hope this helps!
Hope this helps!
do you get the triangle one with tan?
look at the reactivity series
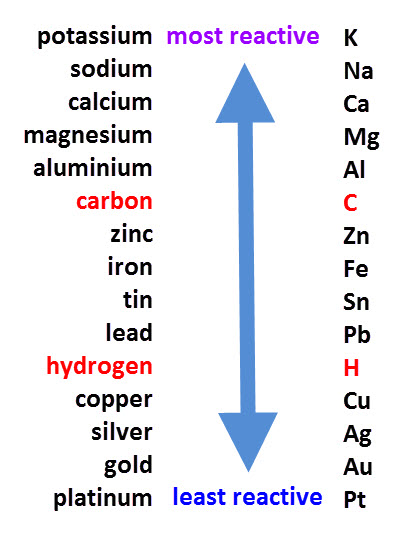

Original post by ilovecake123
AHHH thanks!
any idea where you got this info from cause i wanna do some more reading on this?
any idea where you got this info from cause i wanna do some more reading on this?
Original post by santh1234
look at the reactivity series


do i need to learn this off by heart

sulfur wasnt on here so how did you know it won't displace the iron?
It doesn't have it on the specification but I came up on one of the recent papers I think. I don't know it off by heart yet, just typed it in on google!
Original post by ilovecake123
do i need to learn this off by heart 

*pleaseeeee*
Related discussions
- BMAT Practice Papers
- UCAT Medentry QR Question
- Do I need to do the BMAT to get an interview ?
- Low Ucat advice for med uni
- Bmat cut off
- BMAT - Will a low BMAT score affect your application to non-BMAT universities?
- BMAT Isc medical 700 questions book
- BMAT plz help me
- BMAT Medicine 2024
- is it still worth taking the BMAT
- Oxford medicine ?
- Medicine with 2:2
- Problems accessing my BMAT results
- BMAT 2023 help
- Medicine entry 2024
- Will I be taking the BMAT?
- How should I plan doing both UCAT & BMAT?
- Allied Healthcare Careers with Potential job prospects in UAE
- Bmat scoring
- Is it over?
Latest
Trending
Last reply 1 week ago
OFFICIAL A101 Liverpool University Graduate Entry Medicine Thread 2024 EntryMedicine
77
Trending
Last reply 1 week ago
OFFICIAL A101 Liverpool University Graduate Entry Medicine Thread 2024 EntryMedicine
77



