This discussion is now closed.
Check out other Related discussions
- sister never supports me
- introduction to chemistry
- is studying forensic science a bad idea?
- Biochemistry undergraduate course
- Veterinary medicine with BTEC
- Advanced higher chemistry project help!
- forensic science/veterinary career path
- Help me pick a degree course
- Chemistry EE research question
- A level sciences
- Overall tips for my a level choices
- applying to pharmacy
- Year 12 Work Experience
- Dentistry personal statement
- BTEC applied science level 3
- OCR Chemistry PAG 12.1
- Can I apply for a foundation year with only GCSEs?
- A LEVEL fine art
- Level 3 Extended diploma AM into vet med
- Chemistry degree or dentistry
chemistry investigations
hi Ive been given the idea of doing a chemistry investigation on porphin ring systems, does anyone know anything about them or any suggestions on what I could do? Thanks,
Emma
Emma
Yeah i meant porphyrin (oops)
Well - ideas for chemistry investigations don't immediately come to mind, but do you know what they are?
This site is good:
http://www.washburn.edu/cas/chemistry/sleung/porphyrin/porphyrin_page.html
The most important use of them I can think of though is in Haemoglobin and Myoglobin (oxygen carrying proteins in animals). The prosthetic group in these is a porphyrin ring system with an Fe2+ ion in the middle (it is this that carries the oxygen).
Did your teacher give you any ideas on what to do?
This site is good:
http://www.washburn.edu/cas/chemistry/sleung/porphyrin/porphyrin_page.html
The most important use of them I can think of though is in Haemoglobin and Myoglobin (oxygen carrying proteins in animals). The prosthetic group in these is a porphyrin ring system with an Fe2+ ion in the middle (it is this that carries the oxygen).
Did your teacher give you any ideas on what to do?
I've never heard of porphyrin, seems interesting though.
just looked it up now - haemoglobin works like an octahedral Fe ion complex, with the N and H2O/O2 acting as ligands:
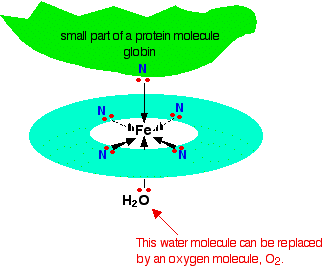
http://www.chemguide.co.uk/inorganic/complexions/whatis.html
just looked it up now - haemoglobin works like an octahedral Fe ion complex, with the N and H2O/O2 acting as ligands:

http://www.chemguide.co.uk/inorganic/complexions/whatis.html
yes, but the 4 planar N atoms marked there are all linked by an aromatic ring system as well, and the top N is part of the protein (from a histidine amino acid side chain).
And I think the porphyrin system is just the ring system containing the 4 Nitrogens ... but it can have an Fe2+ ion put in the middle (and then the N's act as ligands) as it does in Haemoglobin
And I think the porphyrin system is just the ring system containing the 4 Nitrogens ... but it can have an Fe2+ ion put in the middle (and then the N's act as ligands) as it does in Haemoglobin
oops I see now. so in a complex like [Cu(H2O)6] , how are the 4 planar ligands linked together, if they are?
No, here the water molecules are not linked together, but this is NOT a porphyrin. This is just a simple octahedral complex with 6 waters acting as ligands around the central Cu2+ ion.
If you like, think of the porphyrin ring as a polydentate ligand (ie one molecule that can act as a ligand at many points). In fact the porphyrin can bind datively using any of its 4 nitrogen lone pairs (as it does), but H20 on the other hand is a monodentate ligand that can only make one bond in a complex.
If you like, think of the porphyrin ring as a polydentate ligand (ie one molecule that can act as a ligand at many points). In fact the porphyrin can bind datively using any of its 4 nitrogen lone pairs (as it does), but H20 on the other hand is a monodentate ligand that can only make one bond in a complex.
Related discussions
- sister never supports me
- introduction to chemistry
- is studying forensic science a bad idea?
- Biochemistry undergraduate course
- Veterinary medicine with BTEC
- Advanced higher chemistry project help!
- forensic science/veterinary career path
- Help me pick a degree course
- Chemistry EE research question
- A level sciences
- Overall tips for my a level choices
- applying to pharmacy
- Year 12 Work Experience
- Dentistry personal statement
- BTEC applied science level 3
- OCR Chemistry PAG 12.1
- Can I apply for a foundation year with only GCSEs?
- A LEVEL fine art
- Level 3 Extended diploma AM into vet med
- Chemistry degree or dentistry
Latest
Trending
Last reply 2 weeks ago
Unofficial Mark scheme: AQA GCSE Biology Paper 1 Triple Higher Tier 16th May 2023Last reply 1 month ago
AQA A Level Business Paper 3 (7132/3) - 14th June 2023 [Exam Chat]Last reply 1 month ago
AQA A-level Psychology Paper 2 (7182/2) - 25th May 2023 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 1 month ago
Edexcel A Level Mathematics Paper 1 (9MA0 01) - 6th June 2023 [Exam Chat]Maths Exams
2960
Last reply 2 months ago
Edexcel A-level Mathematics Paper 1 [6th June 2023] Unofficial MarkschemeMaths Exams
156
Trending
Last reply 2 weeks ago
Unofficial Mark scheme: AQA GCSE Biology Paper 1 Triple Higher Tier 16th May 2023Last reply 1 month ago
AQA A Level Business Paper 3 (7132/3) - 14th June 2023 [Exam Chat]Last reply 1 month ago
AQA A-level Psychology Paper 2 (7182/2) - 25th May 2023 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 1 month ago
Edexcel A Level Mathematics Paper 1 (9MA0 01) - 6th June 2023 [Exam Chat]Maths Exams
2960
Last reply 2 months ago
Edexcel A-level Mathematics Paper 1 [6th June 2023] Unofficial MarkschemeMaths Exams
156




