Can you have a dialdehyde?
As in can an aldehyde have 2 =O groups, one on each end? Also, if they do exist does that mean there other organic molecules (eg carboxylic acids) can have more than one functional group? (I know alcohols can.)
Thanks
Thanks

Original post by GenericPerson2
As in can an aldehyde have 2 =O groups, one on each end? Also, if they do exist does that mean there other organic molecules (eg carboxylic acids) can have more than one functional group? (I know alcohols can.)
Thanks
Thanks

Hi!
I believe you can have dialdehydes and dicarboxylic acids.
(General formula for a dialdehyde)
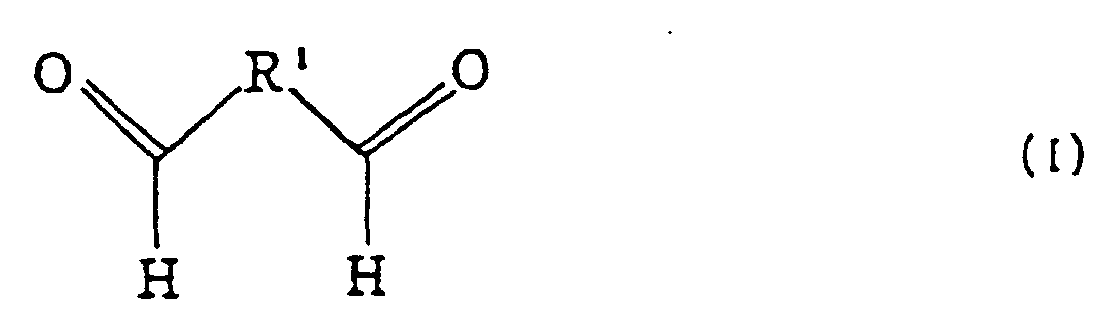
(Propanedioic acid, an example of a dicarboxylic acid)
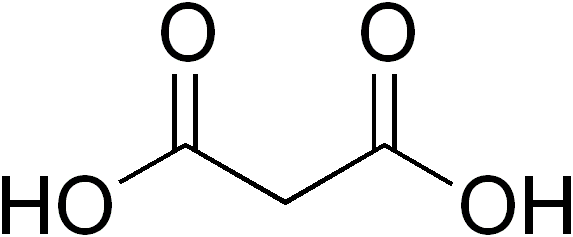
Hope this helped!

Awesome thanks  maybe it'll come up in A2 chemistry...
maybe it'll come up in A2 chemistry...
 maybe it'll come up in A2 chemistry...
maybe it'll come up in A2 chemistry...Original post by GenericPerson2
Awesome thanks  maybe it'll come up in A2 chemistry...
maybe it'll come up in A2 chemistry...
 maybe it'll come up in A2 chemistry...
maybe it'll come up in A2 chemistry...Aha I have actually done practice questions set by my teachers involving them! You never know.

Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- I have a lot OF A-level and GCSE Maths and Further Maths resources. Who's Interested?
- btec science unit 7 part a release 2023 january
- Edexcel IAL Business Studies Notes
- waiting for your thoughts
- Help - considering dropping out of uni
- How do student unions work?
- Economic's AS + A2 Notes (ALL BOARDS)
- can u just walk into a specsavers and try on glasses
- Our tips for attending a Higher Education Fair.
- Part-time Jobs - Boosting your Employability
- DWP Executive Officer Jobcentre working hours
- Dad made me uncomfortable as a child, is this normal?
- As biology aqa paper 1
- University help!
- Uni life
- Losing motivation
- How much
- Can a university revoke my application because of my autism diagnosis
Latest
Trending
Last reply 1 day ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]A-levels
10
Trending
Last reply 1 day ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]A-levels
10




