Primary alcohol
for this the answer is B but makes no sense. surely it’s A as u get the C bonded to two Hs making a primary alcholol
https://share.icloud.com/photos/04j_vw-kMy1XkDcLTRH2r6W8A
https://share.icloud.com/photos/04j_vw-kMy1XkDcLTRH2r6W8A
I'm a little rusty on this but the answer B makes sense to me - wouldn't reducing A form a secondary alcohol rather than a primary alcohol as cyclohexanone is a ketone? Please do correct me if I'm wrong though, I haven't been over this in ages!
Original post by Joey6272
for this the answer is B but makes no sense. surely it’s A as u get the C bonded to two Hs making a primary alcholol
https://share.icloud.com/photos/04j_vw-kMy1XkDcLTRH2r6W8A
https://share.icloud.com/photos/04j_vw-kMy1XkDcLTRH2r6W8A
Reducing A (cyclohexanone) would form cyclohexanol. Draw that out and tell me how many H's are attached to the C with the hydroxyl group.
Reducing B (cyclohexylmethanoic acid) would form cyclohexylmethanol. Draw that one out too.
un
i don’t get this mechanism for B. normally if u reduce that carboxylic acid wouldn’t u change that double bond O to a Oh so there’s two Oh groups.
Original post by Claisen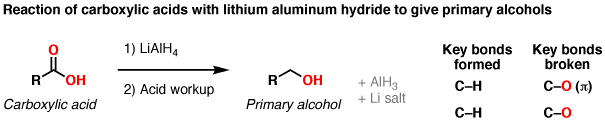
A will give a secondary alcohol. A primary alcohol must mean the OH is terminal. If it is C-(OH)-C then it is secondary.

A will give a secondary alcohol. A primary alcohol must mean the OH is terminal. If it is C-(OH)-C then it is secondary.
i don’t get this mechanism for B. normally if u reduce that carboxylic acid wouldn’t u change that double bond O to a Oh so there’s two Oh groups.
Original post by Pigster
Reducing A (cyclohexanone) would form cyclohexanol. Draw that out and tell me how many H's are attached to the C with the hydroxyl group.
Reducing B (cyclohexylmethanoic acid) would form cyclohexylmethanol. Draw that one out too.
Reducing B (cyclohexylmethanoic acid) would form cyclohexylmethanol. Draw that one out too.
i don’t get this mechanism for B. normally if u reduce that carboxylic acid wouldn’t u change that double bond O to a Oh so there’s two Oh groups.
Original post by Joey6272
i don’t get this mechanism for B. normally if u reduce that carboxylic acid wouldn’t u change that double bond O to a Oh so there’s two Oh groups.
No, not to my knowledge. Google the mechanism (alcohol reduction to acid with LiAlBH4) and you will see what happens. You can use BH3 to isolate the intermediate reactive aldehyde and treat this to give a dialcohol. In reality, with this strong reducing agent, it goes straight to a primary alcohol.
Quick Reply
Related discussions
- A-Level chemistry
- A-Level chemistry
- How do you distinguish between primary and secondary alcohols by chemical reaction?
- using tollens reagent on an alcohol
- CHEM A-level HEL|P
- AQA Chemistry Alevel Organic synthesis
- Urgent ocr a level chemistry!
- Tertiary alcohols and amines
- OCR A-Level Chemistry Synthetic Routes
- Does anyone why the answer to this a level chemistry question is A
- A level organic MC
- Help chem reduction
- A level chemistry optical isomerism MC questions
- Chemistry oxidation of alcohol
- Do you need to know Acylation mechanism for A level OCR chemistry ?
- A level Chemistry OCR HELP
- Most embarrassing thing you ever did when drunk?
- Am I an alcoholic?
- Alcohol withdrawal symptoms
- Female only and alcohol free accommodation
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



