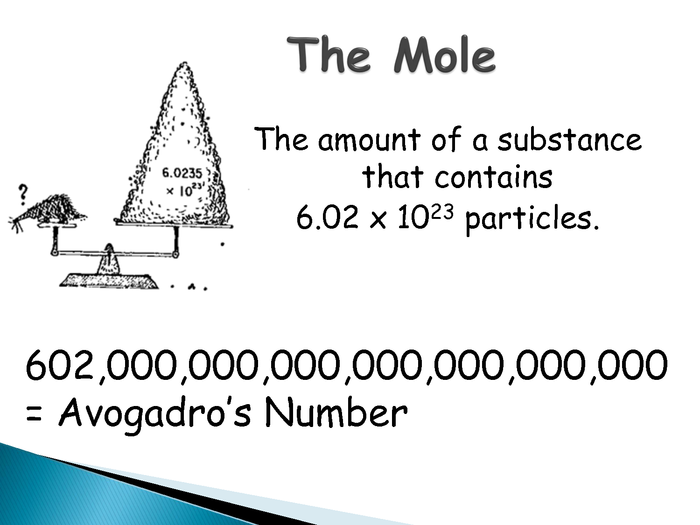
How many atoms in one mole.
Original post by powiful
am i doing it right by dividing it by 1000 or do I need to divide by a different number
if you have 1 x 10^22 atoms, how many moles is that if one mole = 6.022 x 10^23 atoms?
Original post by BTAnonymous
if you have 1 x 10^22 atoms, how many moles is that if one mole = 6.022 x 10^23 atoms?
I have no idea, I seriously hate moles in chemistry 😩
Original post by powiful
I have no idea, I seriously hate moles in chemistry 😩
it clicked trust me. when I was first year I had no idea what was going on, lmao I was so hopeless, you just need to persist.
Remember what a mole is. It's simply just the amount of 'entities' (an entity is any particle; electrons, hydrogen ions, uranium atoms...) we have. One mole has 6.022 X 10^23 entities or 'stuff'.
So if you have 1 X 10^22 of these atoms, then we divide this by Avogadro's number to find the amount of moles we have:
1 X 10^22/6.022 x 10^23.
Can you show us the rest of the question because I'm not sure why you've multiplied by Avogadros?
Original post by BTAnonymous
it clicked trust me. when I was first year I had no idea what was going on, lmao I was so hopeless, you just need to persist.
Remember what a mole is. It's simply just the amount of 'entities' (an entity is any particle; electrons, hydrogen ions, uranium atoms...) we have. One mole has 6.022 X 10^23 entities or 'stuff'.
So if you have 1 X 10^22 of these atoms, then we divide this by Avogadro's number to find the amount of moles we have:
1 X 10^22/6.022 x 10^23.
Can you show us the rest of the question because I'm not sure why you've multiplied by Avogadros?
Remember what a mole is. It's simply just the amount of 'entities' (an entity is any particle; electrons, hydrogen ions, uranium atoms...) we have. One mole has 6.022 X 10^23 entities or 'stuff'.
So if you have 1 X 10^22 of these atoms, then we divide this by Avogadro's number to find the amount of moles we have:
1 X 10^22/6.022 x 10^23.
Can you show us the rest of the question because I'm not sure why you've multiplied by Avogadros?
Looool, thank you! Here’s the full question, I’m stuck on the top two.
(edited 5 years ago)
Original post by powiful
Looool, thank you! Here’s the full question, I’m stuck on the top two. 
yeah, so the equation concentration = mole/volume can be rearranged into concentration * volume = moles.
So whatever the concentration is, multiply this by the volume (in dm^3!! to convert from cm3 to dm3, just divide by 1000).
Quick Reply
Related discussions
- Can someone please explain how to work this out
- Moles
- Can someone please provide me a solution to this
- A-level Transition metal mc question
- Mole calculation
- This question should be easy but I just can't make it make sense
- Equilibrium
- Kc Titration Question
- Chemistry moles help
- chemistry a level ocr question
- Amount of substance q help
- chemistry aqa a level help
- chem help
- Chemistry A Level Titrations Help
- Chemistry - yields question
- Calculate the oxidation state of the vanadium ion?! URGENT
- Titrations
- a level chem help
- hard titration alevel chem Q!
- C3.1.6 Moles, need help!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products