This discussion is now closed.
Check out other Related discussions
- chemistry aqa a level help
- Chemistry alevel aqa amount of substance question
- tof question of calculating mass of one atom
- Chemistry Kc Question
- the total pressure q
- Kc Titration Question
- a level chemistry please help
- Calculating partial pressure
- Confused about Kp and Kc - help please
- how to obtain ratio between gases in decomposition reaction
- Buffer calculation help
- IGCSE Edexcel Chemistry 2023
- Chem alevel help
- Can someone please explain how to work this out
- Can someone please provide me a solution to this
- Moles
- A-level Transition metal mc question
- Equilibrium
- Mole calculation
- This question should be easy but I just can't make it make sense
Mole Fractions
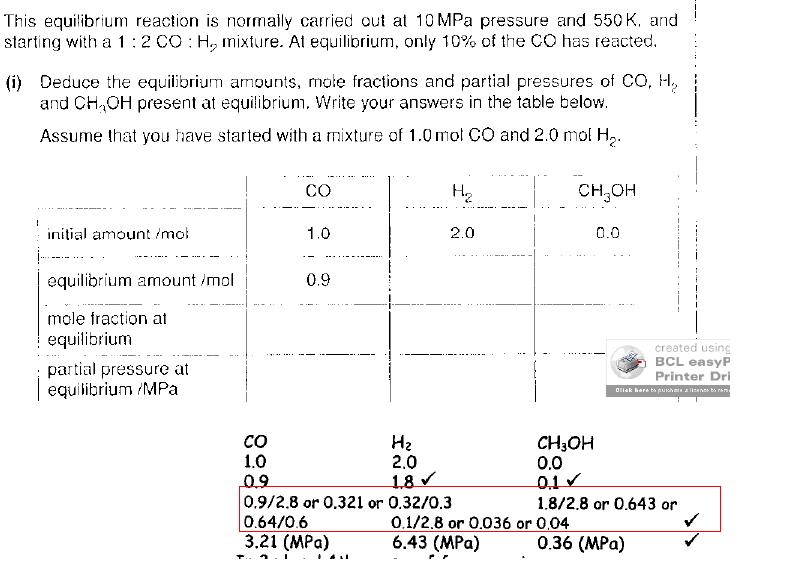
I can do everyhthing the questions asks with no problem, excep the mole fraction part for some reason - taking the example of CO, why is it's mole fraction 0.9/2.8 ? (I know where the 0.9 came from, but where did 2.8 materialise from? - should it now be 3 instead)
TheLoneRanger
I can do everyhthing the questions asks with no problem, excep the mole fraction part for some reason - taking the example of CO, why is it's mole fraction 0.9/2.8 ? (I know where the 0.9 came from, but where did 2.8 materialise from? - should it now be 3 instead)

I can do everyhthing the questions asks with no problem, excep the mole fraction part for some reason - taking the example of CO, why is it's mole fraction 0.9/2.8 ? (I know where the 0.9 came from, but where did 2.8 materialise from? - should it now be 3 instead)
hey, its just the some of all the moles added up.
e.g. 0.9 add 1.8 add 0.1 = 2.8
then divide one of these by this to find the mole fraction.
hope that helped
dunno why they have divided h2 by 3, but the mpa is still right
* Dain Bramaged
hey, its just the some of all the moles added up.
e.g. 0.9 add 1.8 add 0.1 = 2.8
then divide one of these by this to find the mole fraction.
hope that helped
e.g. 0.9 add 1.8 add 0.1 = 2.8
then divide one of these by this to find the mole fraction.
hope that helped
Cheers

I was assuming that the total equilibrium pressure = intial total pressure, guess I should have checked!
Thanks again

* Dain Bramaged
dunno why they have divided h2 by 3, but the mpa is still right
They've just formated the thing a bit weirdly, it says (a bit to the right) 1.8/2.8 which is exactly what you've been saying

Related discussions
- chemistry aqa a level help
- Chemistry alevel aqa amount of substance question
- tof question of calculating mass of one atom
- Chemistry Kc Question
- the total pressure q
- Kc Titration Question
- a level chemistry please help
- Calculating partial pressure
- Confused about Kp and Kc - help please
- how to obtain ratio between gases in decomposition reaction
- Buffer calculation help
- IGCSE Edexcel Chemistry 2023
- Chem alevel help
- Can someone please explain how to work this out
- Can someone please provide me a solution to this
- Moles
- A-level Transition metal mc question
- Equilibrium
- Mole calculation
- This question should be easy but I just can't make it make sense
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products


