SN2 Reaction help
Ok so I was watching a video tutorial about SN2 reaction but one thing is bugging me. In the video, the molecule (nucleophile) that is replacing the leaving group, only part of the molecule does it i.e. in NaCl, just the Cl substitues the leaving group but I've looked on other websites and it shows that the whole molecule, NaCl, substitues the leaving group.
Here's a picutre: Sorry for the bad quality, drew it in paint

Which one is correct, the first mechanism or the second mechanism?
Thanks
Here's a picutre: Sorry for the bad quality, drew it in paint


Which one is correct, the first mechanism or the second mechanism?
Thanks
Original post by mrdoovde1
Ok so I was watching a video tutorial about SN2 reaction but one thing is bugging me. In the video, the molecule (nucleophile) that is replacing the leaving group, only part of the molecule does it i.e. in NaCl, just the Cl substitues the leaving group but I've looked on other websites and it shows that the whole molecule, NaCl, substitues the leaving group.
Here's a picutre: Sorry for the bad quality, drew it in paint

Which one is correct, the first mechanism or the second mechanism?
Thanks
Here's a picutre: Sorry for the bad quality, drew it in paint


Which one is correct, the first mechanism or the second mechanism?
Thanks
Two things....
1. this alkyl halide doesn't undergo Sn2 efficiently, Sn1 is probably the dominating mechanism.
2. NaBr doesn't exist as a discreet molecule. In a solid it is a collection of many (being stupidly large numbers) bromide and sodium ions in a crystal. In solution the crystal breaks down to form sodium and bromide ions in solution which are solvated by the solvent so that they only interact weakly with other ions. The reactive particle in this (ignoring that Sn2 most likely doesn't occur) Sn2 is the Bromide ion in solution, not a NaBr moleucle.
Original post by Exon
Aryl halides don't undergo SN2 mechanisms which is probably why you are getting confused.
eh?? This isn't an aryl halide.
Original post by Plato's Trousers
eh?? This isn't an aryl halide.
Apologies. I was tired and didn't look at the diagram properly.
Original post by Dynamo123
JM has got it right.
he generally does, tbh

Go through sanctioned books of ur academics not internet 4 it. Morrison and boyd is best one,
Posted from TSR Mobile
Posted from TSR Mobile
Original post by Plato's Trousers
he generally does, tbh 


Original post by JMaydom
Two things....
1. this alkyl halide doesn't undergo Sn2 efficiently, Sn1 is probably the dominating mechanism.
2. NaBr doesn't exist as a discreet molecule. In a solid it is a collection of many (being stupidly large numbers) bromide and sodium ions in a crystal. In solution the crystal breaks down to form sodium and bromide ions in solution which are solvated by the solvent so that they only interact weakly with other ions. The reactive particle in this (ignoring that Sn2 most likely doesn't occur) Sn2 is the Bromide ion in solution, not a NaBr moleucle.
1. this alkyl halide doesn't undergo Sn2 efficiently, Sn1 is probably the dominating mechanism.
2. NaBr doesn't exist as a discreet molecule. In a solid it is a collection of many (being stupidly large numbers) bromide and sodium ions in a crystal. In solution the crystal breaks down to form sodium and bromide ions in solution which are solvated by the solvent so that they only interact weakly with other ions. The reactive particle in this (ignoring that Sn2 most likely doesn't occur) Sn2 is the Bromide ion in solution, not a NaBr moleucle.
How come in this reaction:
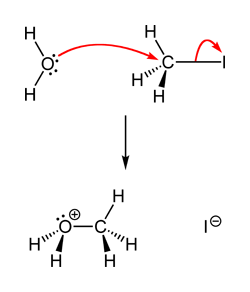 the whole molecule replaces the leaving group as apposed to just the Oxygen?
the whole molecule replaces the leaving group as apposed to just the Oxygen?Original post by mrdoovde1
How come in this reaction:  the whole molecule replaces the leaving group as apposed to just the Oxygen?
the whole molecule replaces the leaving group as apposed to just the Oxygen?
 the whole molecule replaces the leaving group as apposed to just the Oxygen?
the whole molecule replaces the leaving group as apposed to just the Oxygen?In this reaction Oxygen has two lone pairs, and therefore its nucleophilicity allows it to attach the electrophilic carbon whle keeping the other two Hydrogens intact. Since this is a primary AH, therefore, steric hindrance is also not encountered. Comparing to your example of Br-, Br in an ionic compound is not a nucleophile, while O in H2O is one.
Original post by mrdoovde1
How come in this reaction:  the whole molecule replaces the leaving group as apposed to just the Oxygen?
the whole molecule replaces the leaving group as apposed to just the Oxygen?
 the whole molecule replaces the leaving group as apposed to just the Oxygen?
the whole molecule replaces the leaving group as apposed to just the Oxygen?Because the nucleophilc is a molecule. In the NaBr example the nucleophile is the bromide anion. NaBr does not exist as molecules. Hence water substitutes for the leaving group before losing a proton to form the alcohol.
Quick Reply
Related discussions
- SN1 and SN2 reactions (chemistry)
- SN2 nucleophilic substitution -OH
- Electrochemistry help
- Organic chemistry learning a-level
- A level Chemistry Uplearn
- A level chemistry
- Edexcel A Level Chemistry Paper 3 2023
- Oxford Chemistry?
- IAL Chemistry - Unit 4 EXAM DISCUSSION
- Edexcel A-Level Chem Paper 2 Advanced Organic and Physical Chemistry [Exam Chat]
- Ask Us Anything!
- (AQA AS Chemistry) Redox reactions - help!
- equilibriums in reversible reactions
- Factors affecting reaction time
- bio help a level
- Math moments
- AS Chemistry question
- Chemical equations gcse alevel
- Chemistry IA.
- Chemistry Physical question enthalpy of solution
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products


