Original post by Orangee
Hey how would you calculate enthalpy change of formation from combustion data? Please could somebody explain this.
by drawing a hess's law cycle
Original post by dip0
by drawing a hess's law cycle
Original post by Orangee
Hey how would you calculate enthalpy change of formation from combustion data? Please could somebody explain this.
You would use Hess' law. Here is an interactive on Hess' law. Work your way through it and if you still don't understand get back to us ...
Original post by Orangee
and then? I don’t understand this topic at all sorry
so write down the combustion products of the reactants that are in their standard states.
Then go from reactants to the combustion products, then from there to products (but use - the enthalpy change here as we are going from combusted products to the final products rather than the other way around)
Original post by bluepound
The "elements" box is just the reactants in their standard states. Th enthalpy change from this would just be (483.6)+(-393.5)=90.1kj
the -483.6 has to change sign since the arrow is facing up to reactants instead of down
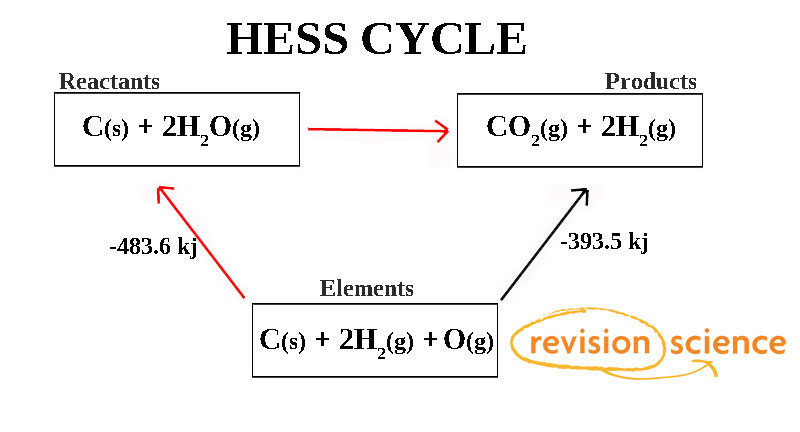
the -483.6 has to change sign since the arrow is facing up to reactants instead of down

This does not actually show combustion enthalpy at all!
Quick Reply
Related discussions
- enthalpy change for combustion?
- Chemistry help pleaseee !!!! 🙏🙏🙏
- enthalpy change of sol more or less exo
- As level chemistry
- Chemistry - solubility and enthalpy of hydration
- A-level chem enthalpy question
- Chemistry question- enthalpy of formation
- Chemistry question- energetics
- Chemistry Olympiad question
- Enthaly Change and Hess's Law
- Enthalpy change
- Bond enthalpies question-help pls
- Enthalpy change question help !!
- 2Al(s) Fe2O3(s) → Al2O3(s) 2Fe(s), struggling with this question
- Chemistry help ( please 😭😭 )
- Hess law and combustion
- enthalpy change of sol
- chem help
- Chemistry ocr a level enthalpy
- Enthalpy change - ocr chemistry - help !!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products




