OCR A2 CHEMISTRY F324 and F325- 14th and 22nd June 2016- OFFICIAL THREAD
Scroll to see replies
Original post by Arima
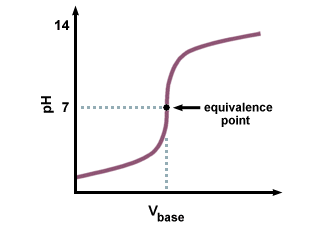
find the pH range of the indicator - say for example it's given as 4.0 - 8.0. Then look at the curve for the acid base titration and spot the end point - so like the centre of the sharp upward line where neutralisation occurs. If the end point lies about the middle of the pH's range (so around 6.0 in this case) then it's suitable
Is the end point the stationary point?
Original post by mechanism
This is what I'm confused about, however I think I get it - when half of HA reacts, that amount of A- has been formed. so the [HA] left = [A-] formed
but if the equilibrium lies so far to the acid side why would so much [A-] be formed?
Original post by Dinasaurus
Is the end point the stationary point?
its bang in the middle of the sharp rise in pH- not sure if it's classified as stationary

Original post by TOMWIGHT
Hi has anyone got the 2015 F325 mark scheme??
K is group 1, so NO3 is -1 charge.
Isn't the only F(VI), FeO4?
Original post by Rust Cohle
Can somebody run through those standard 3 markers on energy comparisons of e.g. RbF vs RbCl or MgCl2 vs NaCl Usually accompanied by a table with some enthalpy values of hydration/solution/lattice enthalpy.
say which one is the smaller ion/has the smaller ionic radius
which one has the higher ionic charge if they're in different groups
say about being more strongly attracted to the oppositely charged ion or to water molecules
say more/less energy energy is required to break the attraction
Original post by tcameron
but if the equilibrium lies so far to the acid side why would so much [A-] be formed?
It's only a half neutralisation when base is added. Remember, it's not just the acid dissociating - it's reacting with the base, so it's no longer true that [H+]=[A-]
Original post by tcameron
say which one is the smaller ion/has the smaller ionic radius
which one has the higher ionic charge if they're in different groups
say about being more strongly attracted to the oppositely charged ion or to water molecules
say more/less energy energy is required to break the attraction
which one has the higher ionic charge if they're in different groups
say about being more strongly attracted to the oppositely charged ion or to water molecules
say more/less energy energy is required to break the attraction
Thanks. If you need any last min assistance quote or pm
Original post by mechanism
This is what I'm confused about, however I think I get it - when half of HA reacts, that amount of A- has been formed. so the [HA] left = [A-] formed
That is pretty convoluted but when you phrase it like that it does make sense.
In answer to your original question I am 99% that is not in the spec, I remember my chem teacher really briefly mentioning it but I'm fairly certain he didn't even go into detail as it was not in the course.
Original post by ToLiveInADream
How would you conduct an experiment to measure enthalpy of solution (4 marks)
Is that an actual exam question? If so, from which year?
Original post by VMD100
That is pretty convoluted but when you phrase it like that it does make sense.
In answer to your original question I am 99% that is not in the spec, I remember my chem teacher really briefly mentioning it but I'm fairly certain he didn't even go into detail as it was not in the course.
In answer to your original question I am 99% that is not in the spec, I remember my chem teacher really briefly mentioning it but I'm fairly certain he didn't even go into detail as it was not in the course.
It's came up before (on several legacy papers), it's on the spec as an application question. Could come up easily. Don't leave anything to chance!
Original post by tcameron
you can't measure the lattice enthalpy directly so how would that work?
You basically measure the temperature change when 1 mole of that substance is dissolved in water . With a thermometer and the conditions , calculate the enthalpy of solution by temperature change times c times , m
Original post by luckycactus1337
You basically measure the temperature change when 1 mole of that substance is dissolved in water . With a thermometer and the conditions , calculate the enthalpy of solution by temperature change times c times , m
so you'd measure the temperature increase or decrease?
For writing ionic equations of say Ca(OH)2 + 2Pyruvic Acid ----> (Pyruvate)2Ca2+ + 2H2O
The ionic equation is apparently just H+ and OH- ----> H2O
Can someone explain how, I don't get why the Pyruvic Acid to Pyruvate isn't also in the ionic equation if it lost a Hydrogen?
The ionic equation is apparently just H+ and OH- ----> H2O
Can someone explain how, I don't get why the Pyruvic Acid to Pyruvate isn't also in the ionic equation if it lost a Hydrogen?
Any examples of dibasic calculations in past papers?
Original post by VMD100
That is pretty convoluted but when you phrase it like that it does make sense.
In answer to your original question I am 99% that is not in the spec, I remember my chem teacher really briefly mentioning it but I'm fairly certain he didn't even go into detail as it was not in the course.
In answer to your original question I am 99% that is not in the spec, I remember my chem teacher really briefly mentioning it but I'm fairly certain he didn't even go into detail as it was not in the course.
Oh okay great thanks!
Anyone got the mark scheme to the 2015 paper?
Original post by Dinasaurus
For writing ionic equations of say Ca(OH)2 + 2Pyruvic Acid ----> (Pyruvate)2Ca2+ + 2H2O
The ionic equation is apparently just H+ and OH- ----> H2O
Can someone explain how, I don't get why the Pyruvic Acid to Pyruvate isn't also in the ionic equation if it lost a Hydrogen?
The ionic equation is apparently just H+ and OH- ----> H2O
Can someone explain how, I don't get why the Pyruvic Acid to Pyruvate isn't also in the ionic equation if it lost a Hydrogen?
cause it hasn't changed oxidation or physical state
Original post by mechanism
cause it hasn't changed oxidation or physical state
surely by losing a Hydrogen it has changed oxidation state.
I still don't really understand the June 2015 42iii question on the adding of the Mg to the buffer :/
In electrode potential
does reduction happen on the positive or negative terminal?
does reduction happen on the positive or negative terminal?
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Go ahead in time as far as possible
- UNIQ 2024 Participants Thread
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- OCR GCSE Geography A Paper 1 (J383/01) - 22nd May 2023 [Exam Chat]
- OCR GCSE Geography B Paper 1 (J384/01) - 22nd May 2023 [Exam Chat]
- OCR A Level Business Paper 3 (H431/03) - 14th June 2023 [Exam Chat]
- OCR A-Level Biology A Paper 2 (H420/02) - 14th June 2024 [Exam Chat]
- OCR B GCSE Chemistry Paper 3 Higher Tier (J258 03) - 22nd May 2023 [Exam Chat]
- Help polymers
- Self-teaching Chemistry A-level (as a private candidate)?
- OCR GCSE Geography Geographical skills J383/03 - 14 Jun 2022 [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Help! How can I improve ?
- How long did you get the offer from Warwick?
Latest
Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



